The Corbevax COVID-19 vaccine was developed by researchers in Texas with no patent, and could be the end of global vaccine inequity.
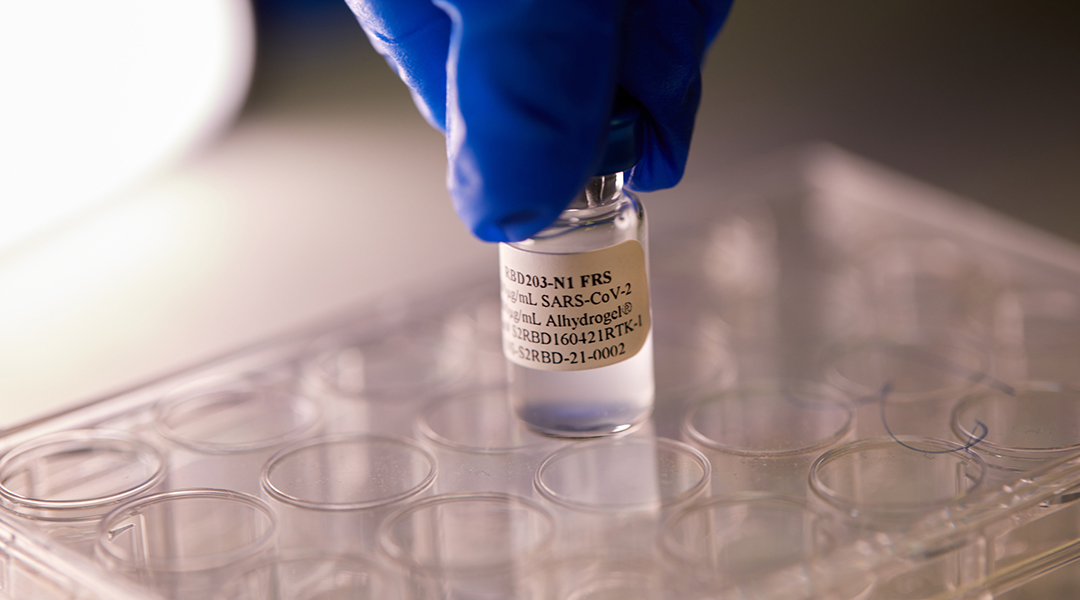

The Corbevax COVID-19 vaccine was developed by researchers in Texas with no patent, and could be the end of global vaccine inequity.

Using materials found around the house, researchers stuck at home created an accurate test to detect COVID-19.
A fast and efficient means of record keeping could improve the ability to trace structural differences in viral variants.

Preliminary data indicates that booster shots improve antibody levels 25-fold compared to just two doses.
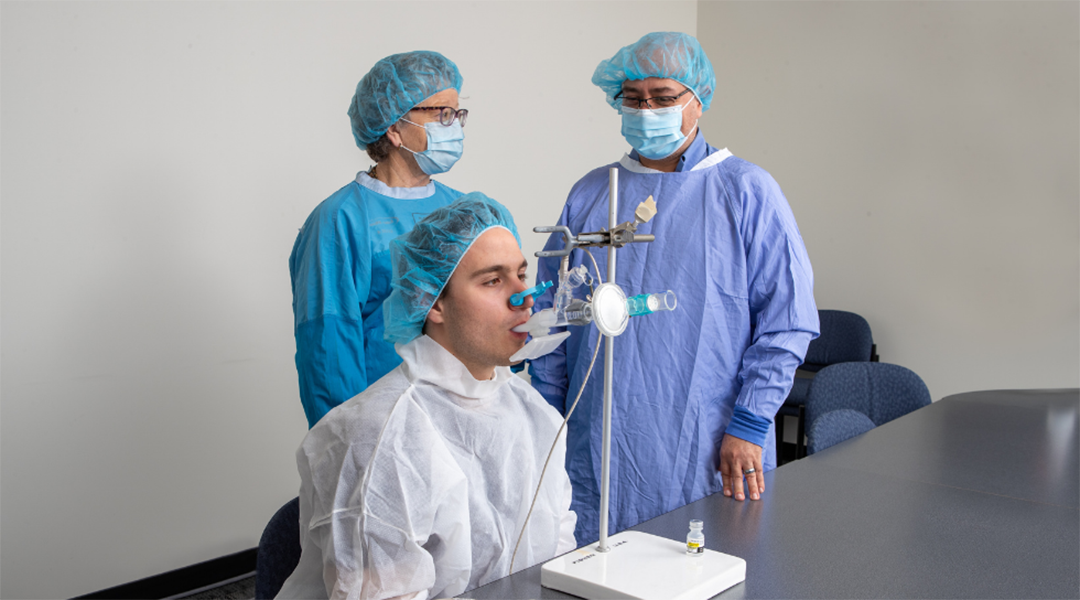
The aerosol platform promises broader protection against SARS-CoV-2, and is currently being trialed as a booster.

Three potent antibodies against SARS-CoV-2 identified in healthy individuals decades ago raises interesting questions about their origins and evolution.
With SARS-CoV-2 continuing to circulate, new oral antivirals promise to be game changers in helping to end the pandemic.
A part of the SARS-CoV-2 spike protein could prove to be its Achilles’ heel.
A biomaterials platform offers a stable center within the body for antibody development against SARS-CoV-2.
An expert review finds that booster shots for the general population are not yet necessary since vaccine efficacy against severe COVID-19 remains high.