Dwindling fossil fuel reserves and environmental concerns are the motivations for a shift to clean renewable energy technologies over the next 50-100 years. Electrocatalytic water splitting into hydrogen and oxygen could potentially address the large-scale energy needs of future societies, assuming efficient and low cost electrocatalysts for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) can be discovered. Ideally, electrocatalysts could be found capable of driving overall water splitting (i.e. HER and OER). However, most electrocatalysts reported to date suffer from high overpotentials for either H2 or O2 evolution, representing a major obstacle to the implementation of this technology.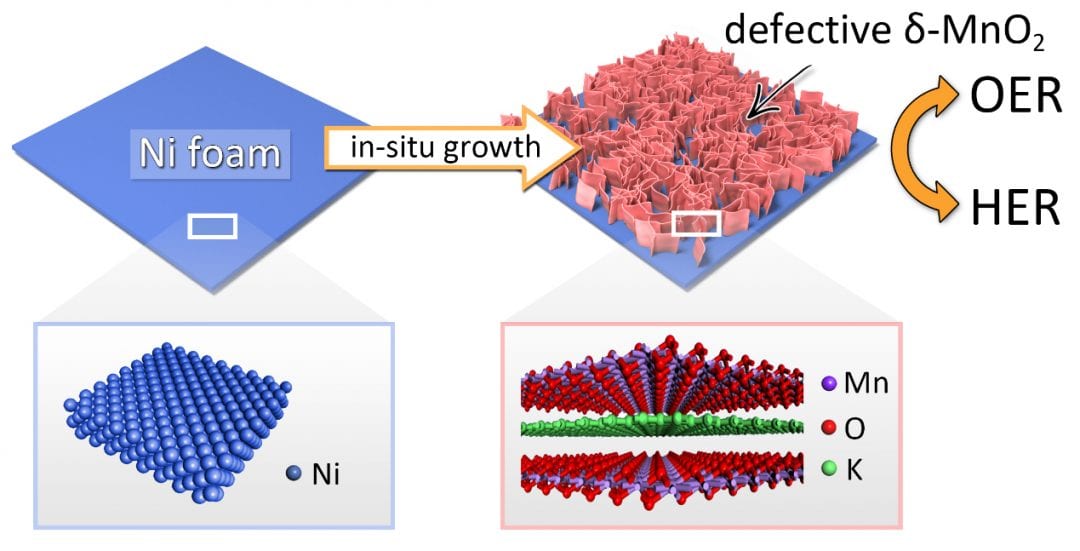
Two-dimensional (2D) ultrathin layered materials constructed from Earth abundant elements are emerging as viable alternatives to conventional Pt, IrO2 and RuO2-based catalysts for water splitting due to their low-cost, excellent activity and high stability. However, many of the 2D layered electrocatalysts reported to date deliver unsatisfactory performance due to their poor electrical conductivity and low concentration of active sites. Novel synthetic strategies are needed to circumvent these particular limitations.
In a recent work, Tierui Zhang (Technical Institute of Physics and Chemistry, Chinese Academy of Science, Beijing), Fei Teng (Nanjing University of Information Science & Technology, Nanjing) and co-workers described a novel synthetic strategy for introducing anion vacancies into semiconductors to significantly enhance their electrical conductivity and electrocatalytic performance. A novel bifunctional electrode consisting of manganese dioxide (δ-MnO2) bilayer nanosheet arrays on nickel foam was prepared using an in situ growth method. The bifunctional electrode exhibited remarkable HER and OER performance, superior to that of commercial Pt/C and IrO2, respectively. The outstanding performance can be attributed to the highly exposed and abundant oxygen vacancies in the d-MnO2 nanosheets, which create Mn3+ active sites that both improve the electrical conductivity of the nanosheets and promote H2O adsorption.
This work highlights the potential of defect-engineering and 2D layered materials in the development of efficient noble-metal-free bifunctional electrodes for electrocatalytic water splitting.