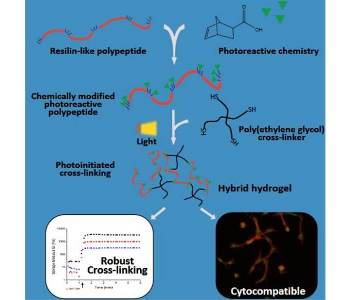
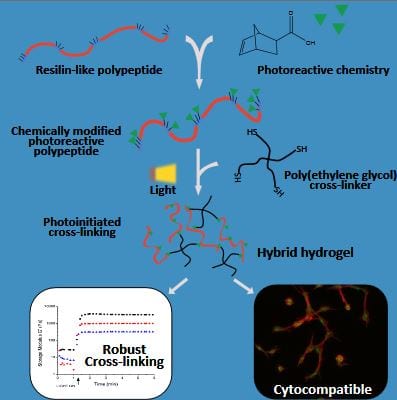
Well-defined biosynthetic polypeptides provide an exciting alternative to traditional naturally-derived or synthetic macromolecules as components in materials for tissue engineering and other applications. Specific bioactivity and desirable mechanical properties are easily imparted to the polypeptides through the implementation of amino acid sequences derived from natural proteins. For example, hydrogels that contain polypeptides modeled after resilin, an elastomeric protein found in insects, exhibit the same outstanding extensibility and energy recovery that is characteristic of the natural protein. While biosynthetic polypeptides thus offer promise as robust, bioactive tissue engineering scaffolds, expansion of the chemistries that can be used to cross-link these materials would offer important opportunities.
Thiol-ene ‘click’ chemistries are particularly useful for producing photocross-linked hydrogels with advantages of showing less sensitivity to oxygen inhibition, excellent cytocompatiblity and homogeneous network formation. The photoreactive chemistries have the added advantage of spatiotemporal control over cross-linking, which permits photopatterning.
Researchers from the University of Delaware introduce a system that harnesses advantages of both biosynthetic polypeptides and photoreactive thiol-ene ‘click’ reactions for the development of hybrid biomaterials for mechanically-demanding tissue engineering applications. Resilin-like polypeptides, expressed in E. coli and functionalized with norbornene acid, were photocross-linked with thiol-terminated PEG macromers to form elastic hydrogels that demonstrated excellent cytocompatibility with human mesenchymal stem cells in both 2D cell-adhesion and 3D photoencapsulation studies. The work demonstrates that this robust photoreactive chemistry can be easily applied to biosynthetic polypeptides, offering new approaches for tailoring these novel materials.