Gut microbiota have a tremendous impact on our physiology and overall health by contributing to metabolic functions, protecting against pathogens, and influencing the immune system. Research in this area is therefore of particular interest, though it seems we have only begun to scratch the surface of this relationship, as more and more studies come to light highlighting the gut’s influence over seemingly unrelated aspects of our health, such as the study that linked gut health with mental well-being.
Most analyses of the gut microbiome rely on fecal DNA and fecal metabolites. However, these methods are often limited because the gut environment is not static.
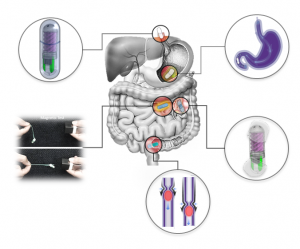
Working principle of the osmotic pill sampler in an enteric capsule.
“Current studies are limited to fecal analysis, which does not provide any information on the spatial distribution of microbial populations in different areas of the gastrointestinal (GI) tract,” says Sameer Sonkusale, one of the authors of the study.
Therefore, in order to gain an accurate representation of the human microbiome, technological advancements which utilize noninvasive sampling techniques at different locations in the GI tract could not only provide new insights into “gut health” but could also provide a better avenue to understanding organ/gut interactions and help to unravel the intricate links between them. In addition, this approach could also advance the development of new therapeutics and treatments that rely on the need to target certain microbiome populations in a specific region of the gut.
An international team of researchers from Tufts University, Universidade Estadual Paulista (UNESP), Utrecht University, and the Biomedical Primate Research Center (Netherlands) sought to address this challenge by developing a non-invasive platform to sample different areas of the gut upstream of the colon in controlled manner.
To accomplish this, the team developed an ingestible 3D‐printed pill with an integrated sampler for in vivo sampling of the gut lumen and its microbiome upstream of the colon. A patient could swallow the pill and receive a complete map of their GI track from the samples the pill collects along the way.
“We [tested] various designs of a biocompatible, ingestible pill that can non-invasively sample the GI tract with more spatial selectivity,” says Sonkusale. “It has a built-in osmotic micropump to draw in the GI fluid into its helical microchannels. No battery or electronics is needed! This pill has the potential to elucidate the composition and metabolic function of intestinal microbial populations and move research beyond what can be accomplished by analyzing fecal material.”
The osmotic pill sampler consists of three main parts; a top sampling head, a semipermeable membrane in the middle, and a bottom salt-chamber (see figure). The head is comprised of four inlets, a stilling basin connected to four helical channels, all of them leading to one small chamber.

Fluorescent feature for easy tracking.
The pill works on the principle of osmosis, where a pressure differential is created across the semipermeable membrane, which creates a passive pumping action. This mechanism facilitates the flow of water across the membrane, which blocks the flow of larger particles (i.e., microorganisms), leaving them trapped in the helical channels.
The pill was enclosed in a pH‐sensitive enteric coating, which prevents it from dissolving in the stomach, and delays sampling until it enters the small intestine, where the coating dissolves under the basic pH environment. An embedded magnet also allows for collection of more sample from a targeted region, and fluorescent tags help to easily identify the device on its way out (image right).
Results show that the bacterial populations recovered from the pills’ helical microfluidic channels in both in vitro and animal model experiments closely resemble the bacterial population of the GI microenvironment.
“By providing information of the spatial distribution of microbial populations in the gut, the pill will revolutionize our understanding of the organ specific host–microbiome interaction, spurring new targeted therapies and treatments for a variety of medical conditions originating from an unbalanced or depleted microbiome,” adds Sonkusale.