Scientists have discovered that the boundary between liquid and metal in a gallium-copper alloy (CuGa2) is less stable than was previously thought, with spontaneous liquefaction of the gallium metal observed for the first time.
“The outer layers of a solidified intermetallic are surprisingly unstable to the depths of several nanometers, fluctuating between a crystalline and a liquid state,” wrote the researchers in their paper published in Advanced Science.
Alloys are blends of different metals, often outshining their individual components in terms of their mechanical, chemical, and thermal properties. “Most alloys begin their journey as a molten metal, which then cools to form a solid,” the team wrote. “The process of solidification is crucial as it dictates the final physical, chemical and mechanical properties, which are profoundly impacted by the final crystalline structure, size, and shape.”
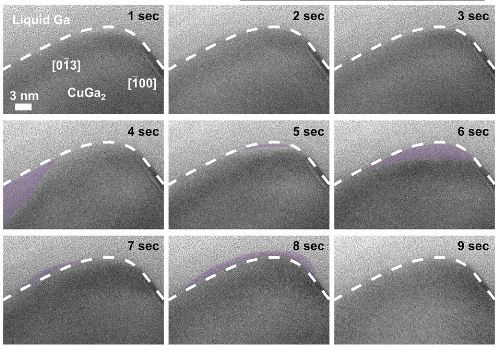
But something unexpected happened when studying the interface between the CuGa2 alloy and liquid gallium (Ga). Atoms of gallium were observed spontaneously transitioning from the solid to the liquid and back again several times per second. This oscillatory movement between states ran dozens of layers of atoms deep.
“This fluctuation of the […] metal surface between solid and liquid phases was completely unexpected because the entire system was being kept close to room-temperature conditions,” said Caiden Parker from the Royal Melbourne Institute of Technology (RMIT), one of the authors of the study, in a press release.
“The liquid gallium ocean was over 200°C colder than the melting point of the Cu–Ga alloy. There would have seemed no possible reason for its surface to keep reverting back to liquid form.”
Blurring the boundary between solid and liquid
Scientists have long studied how alloys form on the surface of solid metal particles that are immersed in liquid metal or a metal with low melting point–the boundary between solid alloy and liquid metal provide intriguing insights.
Using a direct imaging technique called transmission electron microscopy, which uses an electron beam to irradiate the sample and examine the resulting scattering patterns.the team discovered that the interface between the alloy and liquid gallium was much more mobile than expected.
“The outer layers of a solid metal alloy are surprisingly unstable when placed inside a liquid–metal environment, to the depth of several nanometers, fluctuating between crystalline and liquid states,” explained professor Torben Daeneke (also at RMIT), team leader and corresponding author of the study.
Through computational simulations to understand why this was happening, they discovered that near the solid-liquid interface, the energy required for a gallium atom to escape the solid crystalline structure of the alloy was approximately equal to energy needed to remain in place.
This equilibrium led to some of the surface gallium atoms leaving the solid alloy and floating off into the “ocean” of the surrounding liquid gallium. After a few hundred picoseconds, they were followed by copper atoms.
This “escape” of atoms created a vacancy at the surface of the alloy, which ultimately resulted in an instability that led to lattice collapse, causing the liquid-solid interface to retreat inward into the solid.
Then, as the concentration of liquid atoms increased, they began to re-bind with the crystal lattice, which caused the interface to expand into the liquid. This oscillation, where atoms move between the liquid and metal states, occurs within approximately half a second.
Given alloys’ pervasive use in industry, the researchers believe that their discovery will be important not only as a pure scientific endeavor, but will also find applications in technology, although they cannot yet predict exactly where.
“We hope this discovery will open new understanding of how metals behave, for creating new research opportunities, application in new alloy processes, solders, and improved additive manufacturing (3D printing) processes,” concluded Daeneke.
“We can’t know yet what applications this might ultimately lead to,” said Caiden. “We don’t know whether someone will use this new understanding to synthesize improved alloys, or to reduce energy-use in alloy creation, or who knows what.”
Reference: Caiden J. Parker et al, Spontaneous Liquefaction of Solid Metal–Liquid Metal Interfaces in Colloidal Binary Alloys, Advanced Science (2024). DOI: 10.1002/advs.202400147
Feature image credit: ΛΖΞ on Pixabay