Representation of people from diverse backgrounds is imperative to properly assess how a new drug will behave against the genetic, physiological, behavioral, and environmental factors that differ among individuals.
However, achieving inclusivity can be challenging because there is currently a lack of diversity in the cells that most pharmaceutical companies use for drug testing. Previously, studies have not considered the sex of the tested cells as an important variable, for instance, and often there are disproportionately more male-originated cells tested preclinically or males tested in clinical trials, even for diseases shown to affect men and women equally.
This can lead to drugs being approved for use by both men and women without understanding the effects the drug has on different sexes. Robust systems that capture human diversity of sex and many other variables are desperately needed in a lab setting to enhance diversity and inclusion as early as possible in our drug development process.
Kimberly Homan, Director of the Complex in vitro Systems Group at Genentech, and her team envision the long overdue closure of this diversity gap could be achieved by employing a relatively new class of model tissue that uses cells from voluntary donors across different demographics. The models are known as organoids, complex 3D tissues that grow into a miniaturized version of the respective organ and sustain unique traits of their donor.
For Homan and her colleagues, intestinal organoids from donated intestinal tissues, which can be easily obtained during biopsies, are a first step to tackle this challenge — a topic discussed in their perspective published in Advanced Biology.
To achieve diversity, voluntary donation from individuals with different backgrounds is key. Understandably, many marginalized groups harbor deeply rooted medical distrust, and closing the diversity gap must involve continued engagement with these communities.
Homan and her team member, Jessica Klein, sat down with us to explain the fascinating science behind their organoid-based research and how they’re addressing issues of equity and diversity in the field of drug development.
What are human organoids?
Jessica Klein (JK): Organoids are complex 3D tissue models that resemble simplified, miniaturized organs in a dish. They are generated by culturing stem cells in the presence of an extracellular matrix [which acts as a scaffold] and nutrients that reproduce the necessary environmental cues to promote stem cell differentiation into multiple cell or tissue types.
How comparable are human organoid model systems to other currently existing in vitro model systems?
Kim Homan (KH): Organoids add an unprecedented level of complexity over standard tissue culture models, which comprise a single cell type grown on a plastic surface or in liquid suspension, and often immortalized to be maintained indefinitely. These modifications often make these cells more representative of cancer cells than healthy tissue. Such models are very high-throughput and relatively simple to grow and maintain.
Organoids, by contrast, can be more challenging to culture and scale, but they are made up of multiple cell types whose function and interaction more closely resembles the native tissue and whose 3D nature is more representative of human tissue architecture.
How long does the production of human organoids take and can you generate them from any tissue in the human body?
JK: This varies between different tissue and organoid types. Epithelial organoids [epithelial cells form barriers lining inner and outer surfaces of the body] derived directly from adult tissue, such as our intestinal organoids, can take 1-2 weeks to form.
From there, they can be expanded, frozen down, and re-cultured almost indefinitely. These have been generated for many different organ types, and theoretically could be made for any organ that has tissue-resident stem cells (given the right signaling conditions, which can be challenging to mimic).
Organoid complexity can be taken a step further by using induced pluripotent stem cells. These are cells isolated from blood or skin that can be genetically reprogrammed to mimic embryonic stem cells with the potential to grow into any cell/tissue type, generating mini organs containing additional complex tissue, such as vasculature — networks of blood vessels that move blood throughout the body.
However, these can take months to culture and may lose the epigenetic signatures [inheritable changes in gene expression and cell function influenced by the environment that do not modify the DNA sequence] necessary for inclusive studies.
Are organoid model systems the only alternative to currently existing in vitro model systems?
KH: I would be remiss not to mention another popular advanced in vitro model system called organs-on-chip. These are often microfluidic cell culture platforms which simultaneously offer a 3D environment and other cues, such as flow and stretch, locally to human cells.
Organs-on-chip nicely mimic the microenvironment of cells in the body, coaxing those cells to more faithfully model the tissue from which they were derived. Newer methods that combine organoids and organ-on-chip technologies are exciting as they bring the benefits of multiple cell types derived from stem cells together with their microenvironment in a way that starts to approach the complexity found in the human body.
Organoids, therefore, fall somewhere in between the classic traditional 2D models and organs-on-chip systems, offering significant advantages in the ability to model cell diversity while maintaining the ability to use the model at scale in a pharma setting.
Adapting the preclinical drug development process to different demographic groups is important to meet distinctive therapeutic needs. Why is there still such a lack of inclusivity in the early stages of drug development?
KH: There are several reasons for this gap, but two particular aspects to highlight are access to tissue and scale. Traditional in vitro models have gathered cells from single patients, immortalized them, and distributed them among labs. Having a single donor or cell line used amongst multiple labs is great for standardization and reproducibility, but the practice eliminates diversity as a variable.
As the field began to appreciate that donor differences exist, even in vitro, we have worked to include more human cell lines in our preclinical studies. Fairly quickly, however, we realized that often the genetic ancestry and sex of these samples on hand were not as diverse as the population we intend to serve with our therapies.
Genentech [a biotechnology company and independent subsidiary of Roche since 2009] has characterized the genetic ancestry and sex of more than 1,000 parental cell lines in our internal banks to better understand where we fall short of our representation goals. Now we are embarking on a journey to intentionally bank human cells from diverse sectors of the human population, a process which crucially involves patient consent.
Finally, incorporating tissue from more donors into an experiment vastly increases the workload for the scientists. Shifting towards inclusivity in preclinical studies will require investments in automation and scale, taking lab tasks typically done by one scientist with one donor cell line, into an experimental workflow completed by a robot on many human cell lines simultaneously.
How can human organoid model systems capture human diversity and achieve health equity?
JK: Stem cells are the precursor to cell types resident in epithelial tissue, and by culturing them we maintain genetic and epigenetic attributes associated with that individual’s intestine.
By incorporating this diversity preclinically, we can predict whether certain populations will benefit or be harmed by a drug, or investigate unanticipated outcomes of drugs already on the market.
How long can organoids keep the different traits of their donors to guarantee the required diversity in preclinical studies?
KH: While this hasn’t been directly tested yet to our knowledge, scientists have shown that genetic and many epigenetic signatures are largely conserved at reasonable passage numbers [the number of times a cell line has been “reseeded” into daughter cell lines], meaning we can use organoids to model sex and age differences, metabolomic changes, etc.
While these properties certainly need further validation and characterization, using organoids at lower passages (i.e., closer to their initial derivation from tissue) is always advisable. We hope fellow researchers continue to add to the nascent body of literature in this space which will help us further refine the opportunities and limitations of this model system to capture diversity.
How many different adult stem cell-derived human organoid systems are currently being used in preclinical applications?
JK: While there are organoids grown from stem cells for many critical organ systems in the human body, only a handful are currently used in preclinical applications and they are yet to be integrated as a “standard” in the more developmental/translational stages of the drug development pipeline.
One of the main goals of our group is to facilitate adoption of these advanced in vitro model systems (or new approach methodologies (NAMs)) into drug development, and Kim actually just published another perspective that summarizes key considerations for this endeavor.
First, we need to thoroughly characterize our models to understand how their fundamental biology changes in different in vitro environments (aka generate foundational data or model-omics). Secondly, we must qualify these models for their intended use, i.e., conduct context of use (COU) assays. For example, we subject our gastrointestinal (GI) organoids to known GI toxicants and then quantify toxicity to determine the model’s predictive value for clinical outcomes.
Lastly, it’s important that we publish characterization and context of use data to inform model selection for pharma and to answer which model is best for which question. To that effect, we must adhere to FAIR (findable, accessible, interoperable, and reusable) data principles. This means that characterization and context of use data should be shared in repositories that have open access and can be continually mined to compare, validate, and inform the development and use of NAMs.
Who is eligible for donation and how many donors per demographic group are needed for sufficient representation?
KH: For creating biobanks, tissue is generally collected from either a human tissue biopsy taken by a surgeon or an organ donation post-mortem. Anyone can be a tissue donor for biobanking purposes so long as there are no underlying transmissible diseases that would put the personnel handling tissue at risk, such as HIV.
How many donors are needed for representation remains to be discovered and will be based on the specific drug development question at hand. While we don’t have a robust answer yet, Zaaijer and Capes-Davis offer a framework to consider in their 2021 Cell paper about picking cell lines to enhance preclinical diversity.
We hope the community sees these problems as a call to action and helps solve the issue of how to statistically power diversity studies in the preclinical space.
How can potential patients/donors be encouraged to donate their cells and how do you gain the trust of patient groups who have experienced previous abuse by the medical community?
JK: There are several historical examples of individuals and communities exploited for scientific discovery (regardless of intention), however, the scientific community has learned from these mistakes and now operates based off ethical and legal principles in place to provide the patient and/or their family with all information surrounding potential uses of donated tissue and protection against misuse.
While relevant donor information is maintained, it is anonymized to prevent any association with the individual. Education of these concepts, as well as all the scientific and medical breakthroughs enabled from donated tissue, are a big component to gaining trust. To donate tissue, you must be registered as an organ donor, which can be done through your local DMV [in the US] or online at donatelife.com.
This is very quick and easy, and serves as legal consent. When an individual has deceased or is near death, the hospital will contact the local organ procurement organization (OPO) to verify that the individual has consented to organ donation. The OPO is also a great resource to learn more about organ donation.
Do you envision organoid production and in vitro research based on the individual patient to create custom-tailored treatment options in the future?
KH: Personalized medicine is already happening for several diseases. Cystic fibrosis is one such example we mention in our recent paper, where people with specific mutations in the CFTR receptor exhibit differential responsiveness to certain medications.
Personalized medicine is undoubtedly a promising and desirable future state. I am sure organoids will have an increasing role to play in future personalized medicine strategies. In addition, we advocate for bolstering tissue repositories and biobanks to enable predictive studies that identify population-based trends and allow us to classify populations which will respond best to a particular medication.
Reference: Kimberly A. Homan, et al., Toward Inclusivity in Preclinical Drug Development: A Proposition to Start with Intestinal Organoids, Advanced Biology (2023). DOI: 10.1002/adbi.202200333
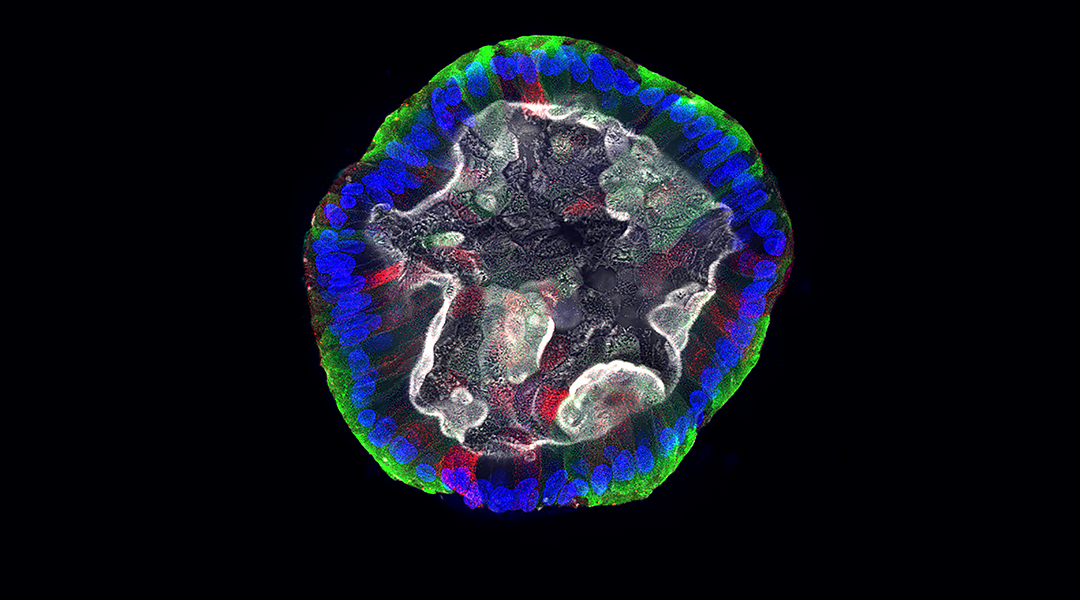
Feature image: Confocal microscopy image of an intestinal organoid showing nuclei in blue, goblet cells in red, enterocytes in green, and actin (which often labels the apical surface) in white. Credit: Jessica Klein