Hydrogen nuclei came into being a mere 379,000 years after the Big Bang, which took place 13.8 billion years ago. However, it took another 400 million years for the universe to sufficiently cool so that these protons could combine with electrons to form the first atoms in our expanding universe.
As this cooling occurred, our beloved Sun evolved from a cloud of gaseous hydrogen undergoing gravitational collapse, eventually reaching a critical point where hydrogen atoms would fuse to form helium and other atoms in a process that would guarantee a steady release of energy for the next 10 billion years. This relatively little ball of molten fusion would later prove to be the source of energy that would sustain life on a rocky ball 150 million kilometers away.
Discovering natural hydrogen reserves
Fast forward to the present, hydrogen makes up 0.14% of the Earth’s crust and we humans are about 10% hydrogen by mass. Earth’s early atmosphere was hydrogen rich, allowing microorganisms to flourish. But, because the planet’s gravitational field is too weak, hydrogen gas is scarcely present in our atmosphere and makes up only 0.00005% of it.
Moreover, because of its high chemical reactivity, it was deemed that it would not be able to exist in gaseous form in large deposits in the Earth’s crust. Instead, it was believed that hydrogen only exists under the rocky planet’s surface when compounded into the structures of hydrocarbon fuels and minerals.
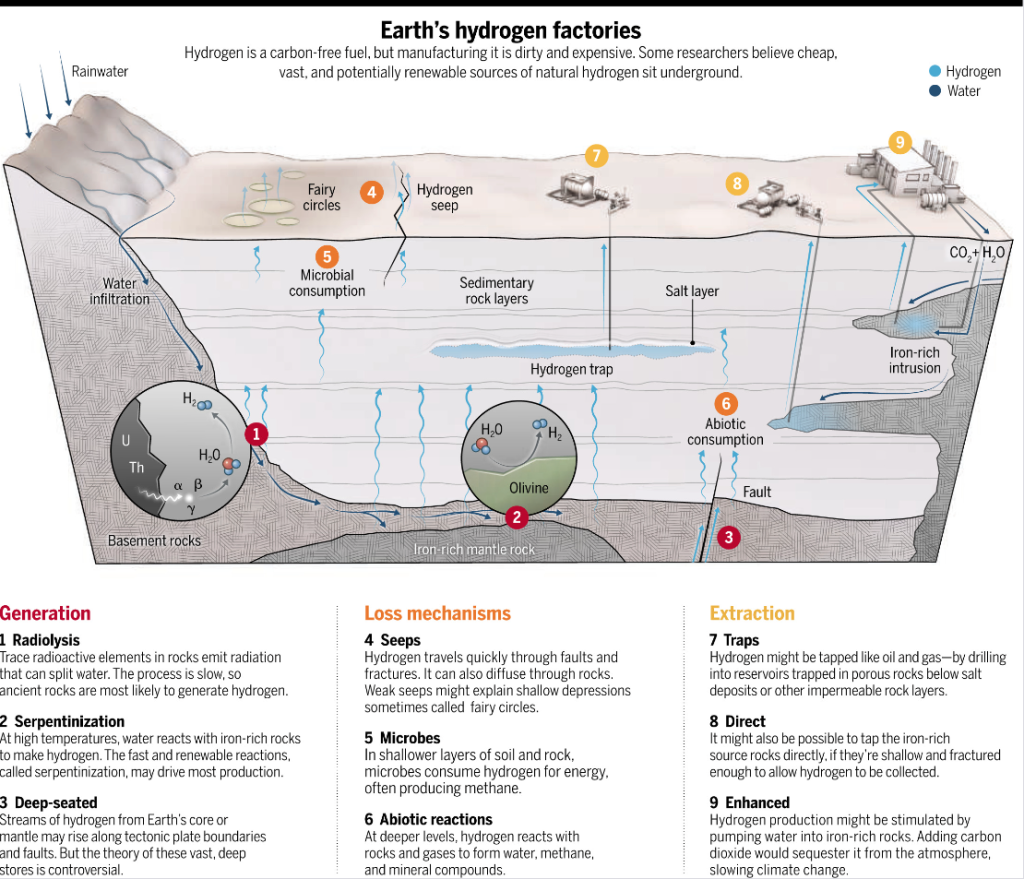
Recent discoveries have unearthed a bonanza of natural hydrogen in significantly larger quantities in the Earth’s mantel than was previously thought possible. Perhaps prejudice against its existence underground combined with the antiquated ways of collecting and analyzing natural gas samples likely explains why its presence, even at concentrations of 10% or more, was missed in known natural gas wells.
Currently, natural hydrogen locations around the world are being identified and drilling is underway for what could be a mighty windfall of Earth-abundant clean hydrogen. This form of natural hydrogen is already being categorized with colors like gold and white to distinguish it from the other sources, such as brown or green, based on their carbon emission intensity.
How is natural hydrogen formed?
It is believed that these natural hydrogen deposits are formed through a process called serpentinization, whereby water reacts with iron-rich mantel rocks containing the minerals olivine and orthopyroxene to form hydrogen gas and a suite of minerals dominated by serpentine. The reaction responsible for hydrogen production can be simplified by the reaction equation:
2(Fe2+O) + H2O → (Fe3+)2O3 + H2
In essence, the lower oxidation state of iron in the minerals olivine and orthopyroxene serves as a reducing agent that converts water to hydrogen. In the process, the iron is oxidized to the higher oxidation and turns into the mineral serpentine.
The hydrogen can then be extracted by drilling into reservoirs formed by porous rocks below impermeable salt or rock layers. If the rocks are sufficiently thin and fractured, it may be feasible to tap them directly for the gas trapped within.
This is an oversimplification of the myriad of processes depicted in the figure that are believed to be occurring in the Earth’s crust. Likewise, there are many factors to consider before drilling for hydrogen. These include where in the planet’s rocky surface would produce the ideal conditions for generation processes, the shapes of the structures trapping the hydrogen, and any possible loss mechanisms in the process.
What could this mean for hydrogen fuel?
It is predicted that the selling price of a kilogram of natural hydrogen could be as low as $0.5, far below the cost of producing hydrogen from either fossil feedstocks or renewables, which sit at $1-3/kg and $3-7.5/kg, respectively. The potential of the natural hydrogen and its promise for disrupting other means of production has not yet been defined in this exciting discovery phase. Numbers like trillions of tons of natural hydrogen are being bandied around amongst explorers, governments, investors, stakeholders, and the media.
Notably, Earth creates natural hydrogen at a much faster rate than it produces hydrocarbon fuels. Furthermore, the fixed volume of a hydrogen reservoir does not control the actual amount of available hydrogen. Rather, gas extracted from deposits is continuously being regenerated by serpentinization processes. Thus, it is not meaningful to think of the size of an in-ground reservoir being a limiting factor in the amount of natural hydrogen available for feeding the future hydrogen economy.
It is amusing to conjecture that if large amounts of natural hydrogen deposits had been discovered at the same time as natural hydrocarbons, would natural hydrogen have served as the preferred feedstock for the ongoing green industrial revolution instead of the fossil-fuel derived hydrogen used today?
One could imagine the availability of natural hydrogen could, for example, have accelerated the development of the hydrogen fuel cell as a source of clean electricity. Likewise, the energy and emission intensities could have been reduced for the Born-Haber and Fischer-Tropsch ammonia and hydrocarbon synthesis processes, respectively, which feed the ever-critical production of fertilizer and transportation fuels. it’ll be interesting to watch how this develops.
Written by Geoffrey Ozin and Jessica Ye
Feature image credit: ben lim on Unsplash

















