 Since the advent of atomic-thickness materials such as graphene, 2D materials are increasingly sought after for use in flexible electronic devices, as electrodes for ultrathin capacitors and batteries, and as highly efficient catalysts. Unlike graphene and other layered materials, it is difficult to generate ultrathin metallic nanosheets, as single layer growth is not preferential, due to their close-packed bulk structure. Using a carbon monoxide confinement technique, researchers in China have managed to fabricate ultrathin rhodium metal nanosheets with thicknesses of less than a nanometer, and controllable area.
Since the advent of atomic-thickness materials such as graphene, 2D materials are increasingly sought after for use in flexible electronic devices, as electrodes for ultrathin capacitors and batteries, and as highly efficient catalysts. Unlike graphene and other layered materials, it is difficult to generate ultrathin metallic nanosheets, as single layer growth is not preferential, due to their close-packed bulk structure. Using a carbon monoxide confinement technique, researchers in China have managed to fabricate ultrathin rhodium metal nanosheets with thicknesses of less than a nanometer, and controllable area.
The method is similar to one used previously by the group to fabricate palladium nanosheets. Firstly, rhodium (II) acetate and an additive, poly(vinylpyrrolidone), are dissolved in N,N-dimethylformamide, charged with CO to a given pressure, then heated to 150 °C. The mixture is maintained at this temperature several hours, during which the Rh nanosheets are formed. In early stages of growth, when the mixture has just reached 150 °C, multiple Rh carbonyl clusters are formed, of 1–2 nm in diameter. These clusters aggregate into Rh nanosheets, reducing the surface energy.
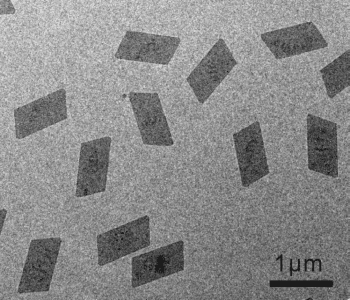
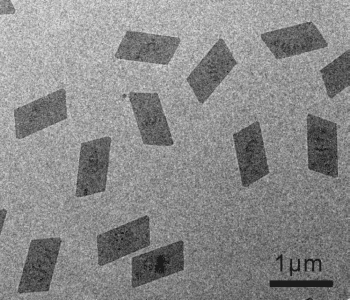
The obtained nanosheets have a uniformly sized parallelogram morphology. The fcc structure of the bulk metal is preserved, but the nanosheets are formed of 3–5 atomic layers of the (111) plane. The individual nanosheets have an average thickness of 0.9 nm, but it was found that 2–5 nanosheets tend to stick together, with a spacing of approximately 0.5 nm between the individual sheets.
The CO confines the growth of the nanosheets to the (111) plane, and prevents 3D Rh structures from forming. Strong CO adsorption onto the (111) plane prohibits further rhodium carbonyl clusters from adhering to this plane, and prevents growth in the [111] direction. Varying the pressure of the CO gas leads to different sizes of nanosheet. Pressured of 0.5, 1, 1.5, and 2 atm give parallelograms with long edges of 500, 800, 1000, and 1300 nm; the ratio of short to long edges is the same at each pressure.
The poly(vinylpyrrolidone) additive acts a surfactant, and, during synthesis, it caps the nanosheets, preventing the formed nanosheets from overlapping. There has been some controversy over whether surfactant cappings enhance or hinder catalytic activity. For the rhodium nanosheets, the best catalytic activity was observed for the non-capped nanosheets in the selective hydrogenation of anthracene, a polycyclic aromatic hydrocarbon.
This growth strategy for metallic nanosheets should also be applicable for other metals, aside from rhodium and palladium. Not only will this lead to reliable and controllable fabrication of metallic nanosheets, but the studies into the effect of surfactant cappings on catalytic activity in a variety of reaction will be easily accessible.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.