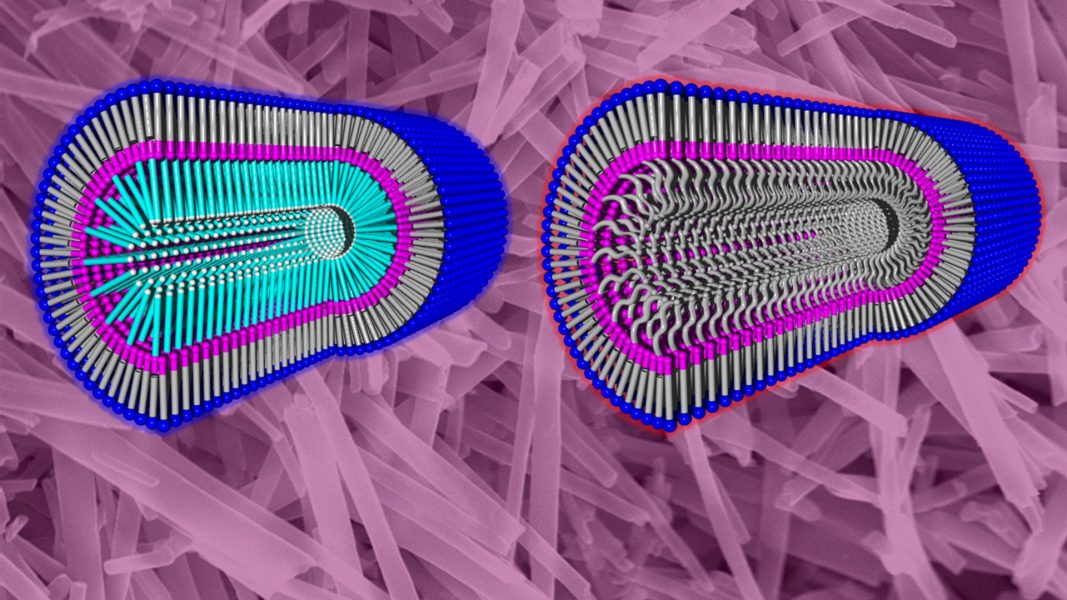
Separation of molecules in solution can be achieved by using liquid chromatography. Chiral molecules, such as the amino acids found in nature and in living beings, are more difficult to separate. Separation via chirally selective nanoparticles in solution could make the process easier.
In their article in Small, Dr. Naohiro Kameta from the National Institute of Advanced Industrial Science and Technology (AIST) in Japan and colleagues demonstrate a series of thermoresponsive poly(ethylene glycol) (PEG)-coated nanotubes for simple and quick in situ separation of chiral molecules.
First, nanotubes were constructed to have a dense layer of short PEG chains on the inner or outer surfaces. Heating solutions of the nanotubes to 60 °C causes the PEG chains to dehydrate and become hydrophobic. When the PEG chains are located on the inner surface of the nanotubes, this creates a hydrophobic channel that can extract amino acids from solution.
Fluorescent-dye-labeled chiral amino acids were used to demonstrate this concept. When added to solutions of the nanotubes and heated to 60 °C, a hypsochromic shift from weak-yellow to strong-green was observed in only a few minutes, confirming the uptake of the amino acids into the nanotubes.
The solutions were then cooled to 25 °C and quencher molecules were added to the solutions to quench the fluorescence of the amino acids upon release from the nanotubes. Using the nanotube inPEG-NT as an example, the L-enantiomers of serine, threonine, cysteine, and tyrosine were released more readily than the D-enantiomers. In contrast, inPEG-NT*, which contains a L-glucose component in the nanotube structure, showed a higher affinity for the L-enantiomers over the D-enantiomers.
Postfunctionalization of the inner surface of the nanotubes with hydrophobic groups endowed them with the ability to separate chiral peptide drugs from solution—up to 100% depending on the number of carbon atoms and steric conformation of the hydrophobic groups.
To find out more about these thermoresponsive nanotubes for the separation of chiral molecules, please visit the Small homepage.