Diabetes is a chronic metabolic disease that results in high blood sugar levels. 422 million adults worldwide have diabetes and 1.5 million people die from it each year (WHO). The most common types of diabetes are a chronic insulin-deficient Type 1 Diabetes (T1D), a chronic insulin-resistant Type 2 Diabetes (T2D), prediabetes, and temporary gestational diabetes.
 T1D is a permanent condition that cannot be cured but can be managed by insulin injections, diet and exercise. T2D and prediabetes can be managed by diet and exercise. With a substantial weight loss, T2D goes away entirely. If poorly managed or left untreated, any form can lead to the severe damage of the heart, kidneys and nerves, blindness, foot amputation, and more.
T1D is a permanent condition that cannot be cured but can be managed by insulin injections, diet and exercise. T2D and prediabetes can be managed by diet and exercise. With a substantial weight loss, T2D goes away entirely. If poorly managed or left untreated, any form can lead to the severe damage of the heart, kidneys and nerves, blindness, foot amputation, and more.
Historically, a urine test was used to measure sugar levels. But it’s a thing of the past. Nowadays, the patients are equipped with the over-the-counter lancet-based digital devices that measure glucose levels in a patient’s blood directly with the built-in enzyme sensors. Yet, their precision, accuracy, and sensitivity come at a steep price – a painful daily ritual, it is far from pleasant and patient friendly.
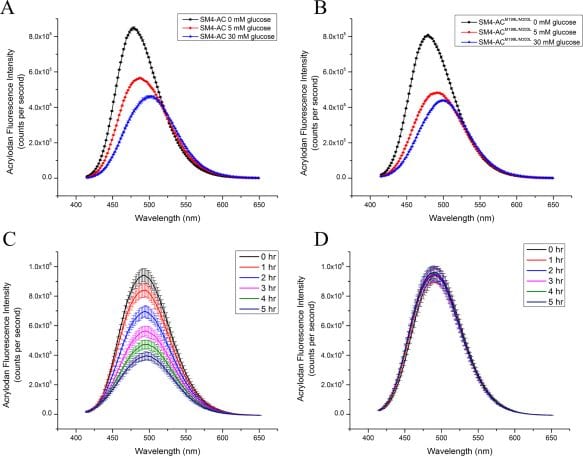
In this Protein Science study David Volkin and colleagues from the Department of Pharmaceutical Chemistry at the University of Kansas, and BD Medical – Diabetes Care report on a comprehensive physicochemical analysis of a prototype continuous glucose monitor developed as a less invasive and more patient-friendly alternative to the lancet based glucose monitors. In this device, a glucose binding protein SMT4 is conjugated to a polarity-sensitive fluorophore acrylodan used for the readings. The in vivo tests of the device revealed a decrease in acrolydan signal, which was attributed to oxidative degradation of SM4-AC. Further H2O2 assay confirmed this finding. The LC-MS peptide mapping analysis identified two Met residues in the device prone to oxidation. The genetic alteration of these residues did not affect the glucose binding affinity of the device, and significantly increased the stability of SM4-AC prototype device in vivo.