 Welcome to one of our guest columns, where active researchers can share their views on topics relevant to materials science. Professor Geoffrey Ozin from the University of Toronto shares his views on the fields of biomimetics and artificial petrification.
Welcome to one of our guest columns, where active researchers can share their views on topics relevant to materials science. Professor Geoffrey Ozin from the University of Toronto shares his views on the fields of biomimetics and artificial petrification.
Materials and structures in nature have successfully evolved, adapted and survived over a 3.5 billion year learning experience. Humankind has always admired and been inspired by the aesthetics of natural form, the visual perception of a class of objects with structural features associated with biological shapes, every one of which has a function and a purpose. Through reverse engineering of nature’s life forms, materials chemists, scientists and engineers have learned how to employ design principles and building strategies that abound in nature’s organisms to find materials solutions to a wide range of scientific and technological problems, from the architectural wonders of the filigree Eiffel tower, Buckminster Fuller’s geodesic dome and Stuttgart airport’s metal tree roof to practical achievements of bone replacement, dry adhesive and military armour.
This is the field of biomimetics (bios: life, mimesis: to imitate, synonymous with bionics, biomimesis, biomimicry, biologically inspired design) and many practical examples now exist of learning from nature’s materials secrets and applying them to solve practical problems for society. The motivation in biomimetics is couched in the question: why reinvent the wheel when nature has spent billions of years finding materials engineering solutions to related problems in biological systems?
In this context, well known bottom-up materials synthesis examples taken from nanobiology include keeping surfaces dry and clean using nanoscale-structured bas-relief patterns analogous to those found on the Lotus leaf, making full color reflective displays based upon the interference of light from a nanophotonic crystal similar to the periodic dielectrics found in butterfly wings, peacock feathers and beetle cuticles, and developing fracture-resistant ceramics by employing layered inorganic–organic nanocomposites of the kind found in the tough calcite–protein laminated architecture of the abalone shell.
I have been ruminating: rather than imitating life forms using nature’s marvels as a blueprint for synthesis from scratch in the incessant search for materials solutions by pursuing biomimicry, why not instead use constructs that abound in the natural world as structure-directing templates for creating copies in synthetic materials selected for particular applications?
In the language of templating, these copies of natural forms can be co-assembled composites or template-free inversions thereof. An exquisite natural example of this is petrified wood where all the organics comprising the hierarchical architecture of wood have been infused with and replaced by silicate minerals (silicification) while maintaining the overall structural integrity of the wood. This is not to be confused with a fossilized impression of wood; rather, it is a three-dimensional representation of the entire internal structure and external form of wood replicated in silicate from the bottom up.
In natural petrification wood is turned into stone using what might be called Medusa chemistry. This kind of morphosynthesis inspires the paradigm of artificial petrification for chemically transforming natural forms into functional inorganic structures with purpose.
Enter artificial petrification, whereby biological structures are employed as templates for making inorganic replicas with purposeful form and function. Laboratory prototypes of this process are beginning to emerge in the literature for solving problems in optics, energy and the environment. Recent demonstrations include the use of siliceous diatom microskeletons as templates for making nanocrystalline silicon diatom replicas with porous lacelike structures conducive to enhanced light-harvesting in solar cells, the use of proteinaceous periodic microstructured wing scales of the iridescent blue Morpho butterfly for templating alumina photonic crystal replicas able to reflect and guide light in miniaturized optical devices, and the use of the hierarchical structure of the green leaf for templating nanocrystalline anatase-platinum replicas for improving the photocatalytic efficiency of splitting water into hydrogen and recycling carbon dioxide into solar fuels like methanol.
Imagine bacteria shapes assembled from silver nanowires and facsimiles of virus cages made of cobalt nanocrystals, calcite coccoliths in graphene and echinoderms in gallium nitride, mosquito eyes in silver and sponge spicules in gold, radiolaria microskeltons in yttrium barium copper oxide and marine diatoms made of copper indium selenide, and the hierarchical structure of Haversian bone in titanium. What would you make out of these?
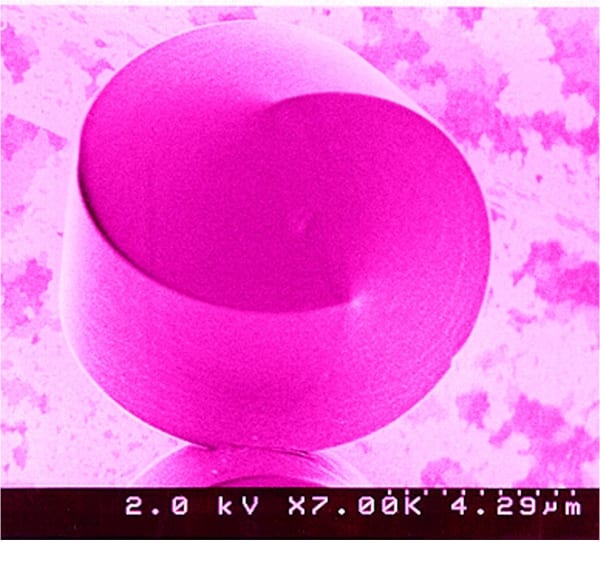
The image above shows an example of morphosynthesis: biomimietic-inspired liquid-crystal-templated, mesostructured silicate. Reproduced with permission from the Canadian Journal of Chemistry, 1999, 77(12), p. 2001.