 Researchers from Japan have developed an innovative and cheap method to produce bi- and trimodal Au–Ag structures and a hierarchical nanoporous Ni–Mn structure. This novel method, which uses Japanese “Washi” paper as a template, is suitable for mass production of electrode materials. Its application leads to the fabrication of the first reported trimodal structure through two-step alloying. Further, the obtained Ni–Mn structure shows excellent electrochemical properties and efficiently catalyzes the oxygen evolution reaction (OER).
Researchers from Japan have developed an innovative and cheap method to produce bi- and trimodal Au–Ag structures and a hierarchical nanoporous Ni–Mn structure. This novel method, which uses Japanese “Washi” paper as a template, is suitable for mass production of electrode materials. Its application leads to the fabrication of the first reported trimodal structure through two-step alloying. Further, the obtained Ni–Mn structure shows excellent electrochemical properties and efficiently catalyzes the oxygen evolution reaction (OER).
Pores of different sizes broaden the functionalities of nanomaterials: Pores in the micrometer range facilitate mass transport such as electrolyte movement. Nanoscale pores enlarge the surface area and, therefore, increase the number of catalytically active sites. Thus, the development of materials with various pore sizes is very important for applications such as supercapacitors, batteries, and electrochemical catalysts.
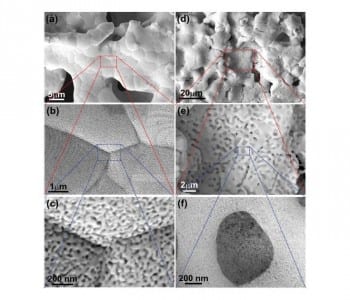
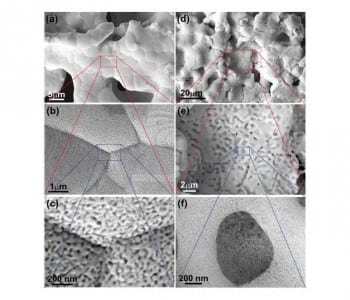
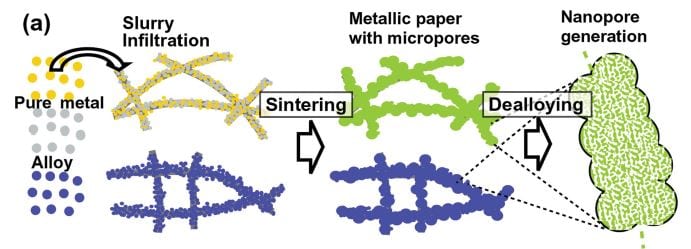 In the method developed by Takeshi Fujita and co-workers, a Japanese “Washi” paper is used as a template. This paper is infiltrated with a slurry of metal powder and then decomposed by sintering, resulting in microporous metal sheets. Nanoscale pores are subsequently formed by etching away the less noble metals in these sheets (dealloying). The trimodal Au–Ag structure is generated by additional annealing and a second etching step. This leads to a nanostructure with pores of three different sizes (10–30 µm, 500 nm, and 20 nm). The cheaper, hierarchical microporous Ni–Mn structure was fabricated with one dealloying step. It has a surface area of 101 m2 g–1, large micropores, and a high capacitance. These properties are responsible for its excellent electrochemical performance and its high OER activity. Further optimization of the fabrication process is still necessary. However, it is a promising approach, which could easily be applied to other alloy systems.
In the method developed by Takeshi Fujita and co-workers, a Japanese “Washi” paper is used as a template. This paper is infiltrated with a slurry of metal powder and then decomposed by sintering, resulting in microporous metal sheets. Nanoscale pores are subsequently formed by etching away the less noble metals in these sheets (dealloying). The trimodal Au–Ag structure is generated by additional annealing and a second etching step. This leads to a nanostructure with pores of three different sizes (10–30 µm, 500 nm, and 20 nm). The cheaper, hierarchical microporous Ni–Mn structure was fabricated with one dealloying step. It has a surface area of 101 m2 g–1, large micropores, and a high capacitance. These properties are responsible for its excellent electrochemical performance and its high OER activity. Further optimization of the fabrication process is still necessary. However, it is a promising approach, which could easily be applied to other alloy systems.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.