Models for toxicological studies are necessarily simplified and, at least for the case of nanoparticles, still largely reliant on observations of two-dimensional cell cultures. Unfortunately, these have a limited ability to reveal the mechanisms of the interactions of analytes with real biological systems.
The increasing applicability of engineered nanoparticles to all manner of technologies right across the research spectrum has seen a push to get them approved for commercial and clinical use, but this requires that they can first be proven safe. In this context, ‘safe’ means that any potential toxicological effects have been assessed and determined not to be a problem for the material’s intended use. But how can we assess this reliably with the current models? Biological systems are not two-dimensional, so the interactions between cells, and between the cells and the extracellular matrix, in a monolayer cell culture will logically be different to the interactions experienced in real biological tissues.
In order to more accurately assess the effects of engineered nanoparticles on real tissue physiology, a group working at the National University of Singapore has used a three-dimensional cell culture model, denoted a ‘3D spheroid model’. This model configuration more closely mimics the physiological complexities of real tissue, providing more realistic and reproducible study scenarios for the interactions of the system with foreign particles.
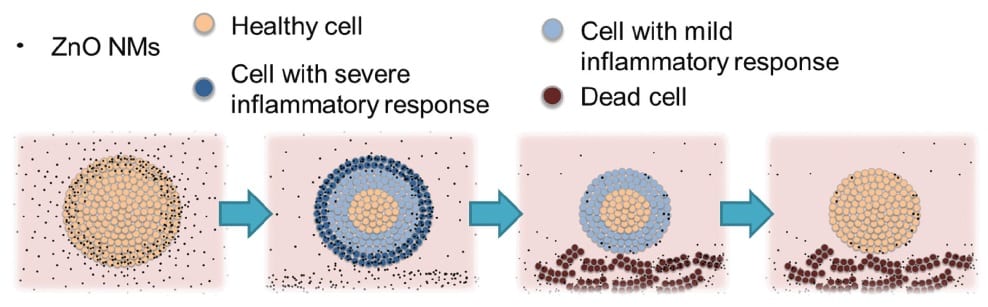
They demonstrate the importance of the 3D nature of the platform by assessing the effects of ZnO nanoparticles on 3D colon cell spheroids. The results indicate that the inflammatory response and cytotoxicity observed are directly linked to the dimensionality of the model: the outer layers of cells acted as sacrificial layers, shielding the cells in the inner layers from any direct toxic effects of the nanoparticles.
It would not have been possible to observe this protective effect using a 2D monolayer culture, where the nanoparticles could cause different modes of cell death. This suggests that the susceptibility of cells in real systems to cytotoxic effects as indicated by such 2D models may be overstated. The mechanisms elucidated by 2D monolayer culture studies may not always be sufficiently indicative of outcomes in real systems, providing strong support for the use of 3D models in future nanotoxicology studies.