
Dr. John Uhlrich, Editor-in-Chief of Energy Technology, talks to Professor Elizabeth Biddinger of The City College of New York, USA about her recent article on copper-based catalysts for carbon dioxide electroreduction, which brings new insights towards the renewable conversion of carbon dioxide, a well-known greenhouse gas, into green fuels and chemicals. It is also included in a Special Issue on Carbon Dioxide Utilization, for which Prof. Biddinger acted as co-guest editor.
- Could you briefly explain the focus and findings of your article to a non-specialist and why they are of current interest?
We are investigating the electrochemical conversion of carbon dioxide to valuable fuels and chemicals. Carbon dioxide, a greenhouse gas and waste product, can be converted to fuels and chemicals electrochemically at room temperature and pressure. There are opportunities to pair excess renewable electricity being generated with carbon dioxide electroreduction to create a sustainable process.
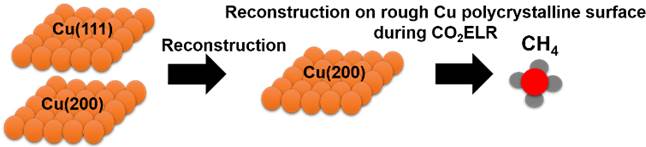
Copper is the only known heterogeneous metal electrocatalyst that can make higher-order carbonaceous products such as methane, ethylene, ethanol, and other C2’s and C3’s (16 possible products have been identified in the literature over copper). The challenge is understanding what the key properties are for the copper electrocatalyst that control the selectivity to a given product. There are well-understood relationships between copper single-crystal electrodes and the resulting products. Unfortunately, the single-crystal work has not translated well for “rough” copper electrocatalysts, which represent more realistic higher-surface-area electrocatalysts.
In our paper, we investigated CO2 electroreduction on rough, electrodeposited copper catalysts. In particular, we observed that catalyst surface reconstruction under CO2 reduction conditions occurs, helping to explain why the work on single-crystal studies does not translate well to more-realistic “rough” catalysts. This copper reconstruction during the reaction changes the morphology and the crystal orientation of the copper. These findings provide insights into what the controlling features are for the selectivity of copper-based electrocatalysts in CO2 electroreduction. If we can create processes that allow for one major product to be formed, the separation costs can be significantly lowered, making the process more commercially attractive.
- Could you please explain the motivation behind the study?
I see CO2 electroreduction as a “hot” area of study for multiple reasons. 1) There has been significant work on carbon dioxide capture in the last 10-15 years. Geologic sequestration of the carbon dioxide was largely seen as the next step of the process after capture. While I think there will still be some sequestration, there is also an opportunity to utilize this waste product as a feedstock. CO2 electroreduction is one such process that can utilize this waste CO2. 2) There has been a significant amount of renewable electricity that has come online in the US and globally recently, especially in the form of wind and solar. These sources are inherently intermittent, making it challenging to manage the overall grid. As a result of this, when it is particularly sunny or windy, we can end up with periods of excess electricity being generated. CO2 electroreduction could utilize this excess electricity to synthesize fuels for use when it is dark (or when the wind isn’t blowing) as a method of chemical energy storage; alternatively valuable chemicals like ethylene could be made during these over-generation periods. Electrochemical processes are more amenable to cyclic operations than thermochemical systems that are generally designed to operate on a continuous basis. 3) There was a great deal of knowledge and skills built in the catalysis and electrochemistry communities regarding fuel cells ten years ago. As a community, we’ve broadened our scope of expertise to electrochemical systems beyond the fuel cell. CO2 electrolysis, while definitely more complicated than a PEM fuel cell, lends itself well to being investigated by this community.
More directly to this particular study, when we were reading the literature on CO2 electroreduction, we found it both exciting and confusing at the same time. There is a lot of great research going on, but also a lack of clarity on why a given copper electrocatalyst will result in a particular product being produced instead of others. We thought we could contribute to providing insights into why a given catalyst results in a particular product being formed. By showing that reconstruction occurs on roughened copper surfaces we can better explain why the roughened catalysts do not follow the same trends as single-crystal surfaces. This work is the beginning of our exploration. We are continuing the studies to better develop our understanding between catalyst morphology and product selectivity.
- How long did this investigation take?
This is our first publication in the area of CO2 electroreduction, so it took longer than normal – approximately 1.5 to 2 years. We started the work while moving into a brand new lab so everything started from scratch. Now that we have everything setup, the research moves much faster. We plan to have a couple more papers on this topic out before the end of this year.
- This work contains largely well-controlled, fundamental electrochemical experiments, but with very practical implications. Do you have a perspective on the role of fundamental research in solving the important challenges that face us today, such as greenhouse gas utilization?
What was the most difficult part about completing this study? Did anything surprise you about your findings?
There is a continuum in research studies from the most fundamental to the most applied, near commercialization. In electrocatalytic studies this goes from using single-crystal electrodes to using more-realistic electrocatalysts in half-cells (studying only the anodic or cathodic reaction), and further on to investigations of full-cells (both the anodic and cathodic reactions together) under realistic operating conditions. The work we are presenting in this study lives in the middle category. While the seminal single-crystal work has provided motivation for the field of CO2 electroreduction, unfortunately there seem to be factors beyond crystal orientation that contribute more strongly to the product selectivity for this reaction. This is why it is so important to study the intermediate step with catalysts that are more realistic. When we study full-cells, elucidating key features of the electrocatalyst becomes more difficult because they cannot be masked by the cell design and operation – both parameters are important to study, ultimately, but don’t allow us to reach the point of overall understanding on their own. Once we have this understanding, we can develop better catalysts that can then be implemented in the full-cells, making them more commercially viable.
For us, I think the most difficult part of the research was going against the seminal work that was done on the fundamental single-crystal electrodes. We have found that the crystal orientation of copper is not the main factor that drives the selectivity on rough, polycrystalline copper catalysts. For example, we were forming methane on rough copper electrodes that from the crystallographic characteristics of the catalyst should have formed ethylene, based upon what the single-crystal studies had predicted. Since this was not what we were expecting, we had to perform additional experiments to support our findings and our claim that the crystallography is not the main factor that impacts the product selectivity on rough copper surfaces. Through a deeper look into the literature, we could see evidence of this as well, though it had not been explained before.
- This work was published as part of a Special Issue of Energy Technology on Carbon Dioxide Utilization, which you co-guest edited with Prof. Hongfei Lin of Washington State University. Towards realizing CO2 utilization on a practical scale, what are some of the most important areas for future development? Have some of these also been addressed in the Special Issue?
First of all, Hongfei and I had the pleasure of having a front-row seat to some really great talks addressing the challenges and solutions to carbon dioxide utilization when we chaired the symposium on “CO2 Utilization” at the Annual Meeting of the American Chemical Society in San Diego in the Spring of 2016. We are excited to be able to share some of that work and more with a broader audience through this special issue of Energy Technology.
Something that stood out for me looking at all of these great works is that there is not one single answer to dealing with carbon dioxide emissions after capture. There are many opportunities beyond sequestration. We particularly highlight thermal, electrochemical, photochemical, and biological methods of CO2 conversion to a variety of useful products. With so many options for how to convert CO2 and a plethora of possible products from these processes, it is difficult to decide where future research and development investments should be made. In my opinion, it would be misguided for us to invest in only one or two of the possible technologies. Like many of the renewable energy and green technologies coming to market, a single solution or process will not be the answer for CO2 utilization. The nature of the CO2 emission source, scale of the utilization process, proximity of chemical plants nearby to use the products, and the ability to integrate with renewable energy sources will all play a role in determining which technology will be the best fit. Two articles in this special issue (Meier, et al., and Navarrete, et al.) address this broader scope of considering how the CO2 utilization fits into these considerations. Interestingly, both of these articles highlight the importance of renewable energy sources being tied to CO2 utilization.
- How will you follow up on this work?
For us, the driving force of us remains the same – the improvement of selectivity and activity of CO2 electroreduction using copper electrocatalysts. We are currently investigating what electrocatalytic factors impact product selectivity beyond crystal orientation. By identifying the key characteristics that impact the selectivity we will be able to design and synthesize catalysts for desired products – something that will assist in bringing CO2 electroreduction to the commercialization phase. We’ve figured out how to tune the rough copper catalyst to switch between methane and ethylene as the primary product, and we are working to clearly understand what the key property differences of the catalyst are, as it does not seem to be copper crystal orientation.














