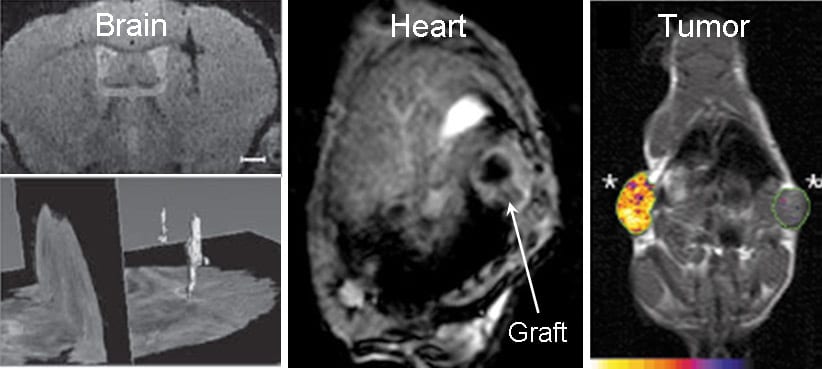
In order to image transplanted cells, they have to be labeled with a contrast agent that is detectable with a specific imaging modality. There are two main approaches to label cells: either by tagging them with an artificial contrast agent carrier system (for example, iron-oxide nanoparticles) or by modification of cellular DNA to induce expression of specific contrast-enhancing proteins (imaging reporter genes) that also increase iron content of cells. Artificial nanoparticles provide a strong signal on MRI and enable high-resolution visualization of the migration and homing of injected cells, but are less suitable for studies of cell survival in the longer term. Alternatively, genetically encoded iron-associated cell labeling systems enable reliable assessment of long-term graft survival and proliferation.
Despite the obvious advantage to monitoring cells for the longer term, the use of iron-associated MRI reporters still faces challenges regarding sensitivity of MRI compared to other modalities, as well as signal specificity issues. Novel molecular cloning techniques, such as zinc finger nuclease (ZFN), CRISPR, and TALENs enable targeted genome addition and provide persistent expression of the transgene while avoiding gene silencing and insertional mutagenesis caused by viral vector-mediated random integration. A recent review article in WIREs Nanomedicine and Nanobiotechnology summarizes the current state-of-the art, providing insight into the pros and cons of existing technology, and also discusses the future directions for molecular imaging and techniques for improving sensitivity, specificity, and safety of in vivo reporter gene imaging and the potential for transition into clinical trials and practice.
Kindly contributed by the authors.