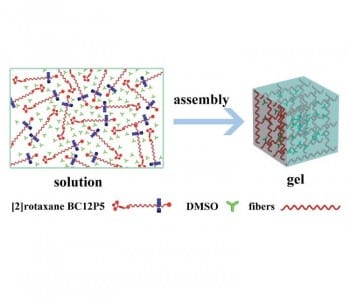
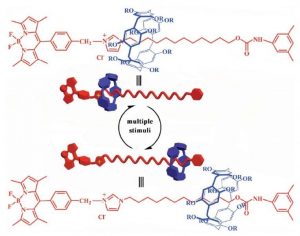
 Recently, researchers from Beijing and Guizhou designed a rotaxane with a mechanically interlocked structure, which is sensitive to various external stimuli. The pillar[5]arene wheel of [2]rotaxane BC12P5 moves along the dumb-bell-shaped axle upon changing temperature, pH, and solvent polarity. This qualifies the rotaxane as a molecular shuttle, which is fluorescent because of its boron dipyrromethene (Bodipy) stopper. Additionally, the rotaxane forms a fluorescent gel, which is sensitive to external stimuli. As a consequence, the gel is able to act as a pH-sensitive gas sensor.
Recently, researchers from Beijing and Guizhou designed a rotaxane with a mechanically interlocked structure, which is sensitive to various external stimuli. The pillar[5]arene wheel of [2]rotaxane BC12P5 moves along the dumb-bell-shaped axle upon changing temperature, pH, and solvent polarity. This qualifies the rotaxane as a molecular shuttle, which is fluorescent because of its boron dipyrromethene (Bodipy) stopper. Additionally, the rotaxane forms a fluorescent gel, which is sensitive to external stimuli. As a consequence, the gel is able to act as a pH-sensitive gas sensor.
Rotaxanes are widely used building blocks for supramolecular structures such as molecular shuttles and switches. Using a fluorescent Bodipy moiety as a stopper opens up their structure for applications as optical sensors. The mechanically interlocked structures of rotaxanes are also able to act as gelators for supramolecular gels. Van der Waals forces between the alkyl chains of the axles and π–π interactions between phenylene stoppers lead to the formation of self-assembled networks.
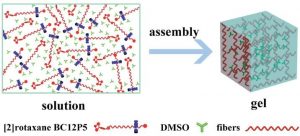 Nana Sun and colleagues explored the reaction of both the dissolved rotaxane and the gel toward external stimuli. The pillar[5]arene wheel moved towards the Bodipy stopper, when the temperature of the solution was increased and when the polarity of the solvent was decreased. The fluorescence intensity of the dissolved rotaxane was manipulated by changing the pH and the temperature of the solution. Nevertheless, these fluorescence properties are only slightly changed by the movement of the wheel.
Nana Sun and colleagues explored the reaction of both the dissolved rotaxane and the gel toward external stimuli. The pillar[5]arene wheel moved towards the Bodipy stopper, when the temperature of the solution was increased and when the polarity of the solvent was decreased. The fluorescence intensity of the dissolved rotaxane was manipulated by changing the pH and the temperature of the solution. Nevertheless, these fluorescence properties are only slightly changed by the movement of the wheel.
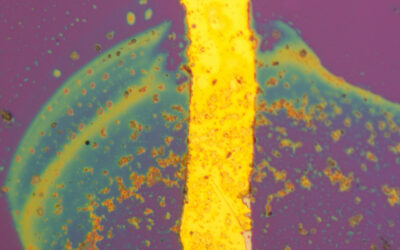
The gel can be turned into a liquid by shaking, heating, and by adding anions. This process is reversible, since resting, cooling and adding cations leads to gel reformation. Exposing the gel to hydrogen chloride gas changes the fluorescence color from yellow to purplish red and decreases the intensity, while ammonia gas increases the intensity but does not change the color.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.