This article is an invited piece from Professor Geoffrey Ozin, University of Toronto, on his 2015 RSC Centenary Award for his work in defining, enabling and popularising a chemical approach to nanomaterials for innovative nanotechnology in advanced materials and biomedical science
Where Did It All Begin?
In this Perspective I will look back over my careers work and reminisce, with the help of a few graphical depictions, about the “eureka moments” that led me to imagine and help develop the field of Nanochemistry.
 In the early seventies, the century-old field of colloid chemistry, pioneered by Thomas Graham (1805-1869), was undergoing its metamorphosis into today’s Nanochemistry. Colloid science, propagated by Wolfgang Ostwald (1883-1943), was nucleating as today’s Nanoscience. Graham had described the distinctive behavior of matter in the nanometer to micron size range, and Ostwald had enriched the subject in his book ‘The World of Neglected Dimensions’(1914). This work inspired me as a new Assistant Professor arriving in the Chemistry Department at the University of Toronto in the summer of 1969, to address the challenge of making materials with nanoscale dimensions using a bottom-up chemical approach. As a synthetic chemist, I was confronted at that time with an important unanswered question: How could chemistry be used to prepare nanoscale forms of well-known metals, semiconductors and insulators having physical dimensions in the quantum size regime of around 1-100 nm with control over their size? The dream was to study the size-tunable chemical and physical properties of these materials with an eye to elucidating function and ultimately to determine utility in a number of perceived applications that would benefit from the ‘nano advantage’.
In the early seventies, the century-old field of colloid chemistry, pioneered by Thomas Graham (1805-1869), was undergoing its metamorphosis into today’s Nanochemistry. Colloid science, propagated by Wolfgang Ostwald (1883-1943), was nucleating as today’s Nanoscience. Graham had described the distinctive behavior of matter in the nanometer to micron size range, and Ostwald had enriched the subject in his book ‘The World of Neglected Dimensions’(1914). This work inspired me as a new Assistant Professor arriving in the Chemistry Department at the University of Toronto in the summer of 1969, to address the challenge of making materials with nanoscale dimensions using a bottom-up chemical approach. As a synthetic chemist, I was confronted at that time with an important unanswered question: How could chemistry be used to prepare nanoscale forms of well-known metals, semiconductors and insulators having physical dimensions in the quantum size regime of around 1-100 nm with control over their size? The dream was to study the size-tunable chemical and physical properties of these materials with an eye to elucidating function and ultimately to determine utility in a number of perceived applications that would benefit from the ‘nano advantage’.
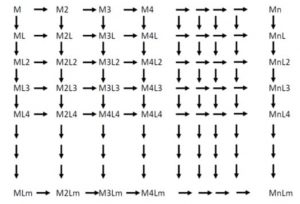 My first ‘eureka’ moment arose by performing chemistry with ‘naked’ metal atoms under cryogenic conditions, where chemical reactions could be ‘stopped in their tracks’ and reactive intermediates and products could be observed by various forms of spectroscopy. This revelation opened my mind to the tantalizing possibility that one could control nucleation and growth of metal atoms, one atom at a time, to form ‘atom-precise’ metal nanoclusters Mn, by allowing them to diffuse around, self-assemble and become immobilized in low-temperature inert and reactive solid matrices. This scientifically exciting feat had never been accomplished before. In this way, I was able to observe metal-atom-by-metal atom nucleation and growth reactions, and monitor and quantify the formation of Mn and define their aggregation kinetics. Furthermore, I established that it was possible to observe previously unforeseen MnLm compounds from reactions of these naked metal atoms and metal clusters with a wide range of small molecule ligands exemplified by L = CO, N2 and O2 (Nature 1972, Nature 1975).
My first ‘eureka’ moment arose by performing chemistry with ‘naked’ metal atoms under cryogenic conditions, where chemical reactions could be ‘stopped in their tracks’ and reactive intermediates and products could be observed by various forms of spectroscopy. This revelation opened my mind to the tantalizing possibility that one could control nucleation and growth of metal atoms, one atom at a time, to form ‘atom-precise’ metal nanoclusters Mn, by allowing them to diffuse around, self-assemble and become immobilized in low-temperature inert and reactive solid matrices. This scientifically exciting feat had never been accomplished before. In this way, I was able to observe metal-atom-by-metal atom nucleation and growth reactions, and monitor and quantify the formation of Mn and define their aggregation kinetics. Furthermore, I established that it was possible to observe previously unforeseen MnLm compounds from reactions of these naked metal atoms and metal clusters with a wide range of small molecule ligands exemplified by L = CO, N2 and O2 (Nature 1972, Nature 1975).
One of my favorite early initiatives, undertaken while working as a Fairchild Fellow at Caltech in 1977 with William Goddard, was an experimental and theoretical study of Nin(C2H4)m. This described for the first time the chemistry of ‘naked’ nickel atoms and nickel clusters with ethylene, envisioning them as a localized bonding model for ethylene chemisorbed on bulk nickel (JACS 1978). The ingenuity behind these 1970’s experiments, summarized in ACR 1973 and ACR 1977, unveiled an unprecedented view of controlled size metal nanoclusters, the synthesis and study of which enabled the first explorations of the transition from molecular to quantum confined to bulk forms of metals (JACS 1980). They also provided a unique platform for investigating cluster-surface relations, chemisorption models and modelling them theoretically.
It is worth mentioning that I later expanded and enriched this work with the discovery of a collection of unprecedented metal atom and metal cluster photo-processes. These light induced processes included ‘naked’ metal atom photo-aggregation, ‘naked’ metal cluster photo-dissociation and ‘naked’ metal cluster photo-isomerization reactions as well as ‘naked’ metal atom-molecule photo-insertion reactions. The latter included the hydrogen-hydrogen bond of di-hydrogen and the carbon-hydrogen bonds of saturated hydrocarbons, such as methane to make binary MH2 and HMCH3 archetypes for many of the first transition series elements (ACIE 1983, 1986, JACS 1985). Together, these early experiments on the chemistry and photochemistry of ‘naked’ metal atoms and ‘naked’ metal clusters, were beginning to help lay the groundwork for the development of Nanochemistry as we know it today.
Zeeing Zeolite
My desire to take the insights gained from this phase of my Nanochemistry work on ‘naked’ metal atom and ‘naked’ metal cluster cryochemistry, ‘out of the cold’, provided the link between my early work and the field of zeolite science. I envisioned making and stabilizing these tiny pieces of matter so that detailed studies of their structure, property, function and utility could be undertaken. In this context, it occurred to me that because these Mn and MnLm nanoclusters were inherently metastable with respect to further agglomeration to thermodynamically stable bulk materials, they had to be stabilized by some kind of surface protecting sheath. I performed the nucleation and growth reactions within the nanometer-sized voids of zeolites, ‘capping and trapping’ the nanoclusters in a ‘zeolate’ ligand cage (AM 2004). This work confirmed that zeolites could serve as nanoporous hosts for synthesizing and stabilizing metal and semiconductor nanomaterials.
During this period, thinking within the zeolite community focused solely on the properties and applications of zeolites in catalysis and gas separation. However, I preferred to look at zeolites as solids filled with periodic arrays of nanoscale voids and wondered how they could perform and compete in the advanced materials research space. I saw their potential in areas such as information storage, photovoltaics, batteries, fuel cells, photo-catalysis, chemical sensors and drug delivery systems. Exploring this potential, I worked with Edith Flanigen at Union Carbide, Tarrytown New York for five years to bring some of these ideas to practice, ultimately describing a vision for the future direction of the field in my paper ‘Advanced Zeolite Materials Science’ (ACIE 1989).
Coincidentally, around this time the Union Carbide team made the extraordinary discovery that nanoporous materials could be made from elements across the periodic table, thus expanding the composition field of zeolites way beyond aluminosilicates and silicates. This advance inspired me to focus my attention on advanced materials applications of nanoporous metal chalcogenides, which I envisioned as semiconductors filled with nanometer holes (Nature 1997) with perceived utility in molecular size- and shape-discriminating sensing devices enabling the development of an early ‘electronic nose’, (SC 1995).
Escape from the 1 nm prison
The 1 nm-size voids in zeolite hosts imposed on their imbibed guests presented an impediment to my quest to nucleate, grow, stabilize and study quantum-confined nanomaterials with physical dimensions in the 1-100 nm range. This length scale was defined by quantum physics, which necessitated larger voids than those offered by zeolites. It was Charles Kresge and co-workers 1989 discovery at Mobil Research, New Jersey of periodic mesoporous silica materials with nanometer tunable 2-100 nm voids that enabled me to break free from the 1 nm prison of zeolites.
Birth of Nanochemistry
My ensuing research laid out the essence of a chemical approach to nanomaterials – a futuristic field that I called ‘Nanochemistry’, (AM 1992). This paper set the scene for a nanomaterials revolution that continues unabated today. In this paper I envisioned the novel world of Nanochemistry with its 0-D dots, 1-D wires, 2-D layers and 3-D open frames, configurations that surprised, shape- and size-dependent behaviors that startled. Here were the conceptual foundations, the description of a bottom-up paradigm for synthesizing nanoscale materials with nanometer-level command over their size, shape, surface and self-assembly. The potential I saw was breathtaking. It would be possible to produce nanoscale materials – perfect down to the last atom – from organic and inorganic components, with structure-property relations designed to yield new materials characterized by an array of novel behaviors and these materials would have real-world applications.
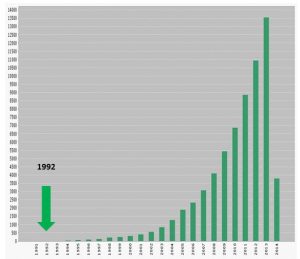 The field of Nanochemistry crystallized in 1992 and gave birth to an explosion of journals that publish Nanochemistry with citation impact-factors matching or exceeding those published in the flagship journal of their respective society. These include: Nano Letters, ACS Nano, Nature Nanotechnology, Nanoscale, Small and the list continues to grow. Chemistry and Nanotechnology were forever united through Nanochemistry, evidenced by the astronomical growth of Nano Chemistry ISI citations since 1992, more than 80M hits on Google, and the creation of numerous global initiatives in academic, industry, government, and defense institutions around research and education in Nanochemistry. It is likely that these initiatives would not have been possible without the key role that my research group played in developing the early phase of the field in the seventies – a contribution which subsequently inspired others to employ fundamental scientific principles and practices of Nanochemistry to solve challenging real world problems in Nanotechnology.
The field of Nanochemistry crystallized in 1992 and gave birth to an explosion of journals that publish Nanochemistry with citation impact-factors matching or exceeding those published in the flagship journal of their respective society. These include: Nano Letters, ACS Nano, Nature Nanotechnology, Nanoscale, Small and the list continues to grow. Chemistry and Nanotechnology were forever united through Nanochemistry, evidenced by the astronomical growth of Nano Chemistry ISI citations since 1992, more than 80M hits on Google, and the creation of numerous global initiatives in academic, industry, government, and defense institutions around research and education in Nanochemistry. It is likely that these initiatives would not have been possible without the key role that my research group played in developing the early phase of the field in the seventies – a contribution which subsequently inspired others to employ fundamental scientific principles and practices of Nanochemistry to solve challenging real world problems in Nanotechnology.
Micro-, Meso-, Macro-Scale
This work mapped the foundation for much of my research on nanomaterials which underpinned my ‘panoscopic’ vision of materials self-assembly over ‘all’ length scales (ChemComm 1999), a synopsis of which is described below with graphical illustrations.
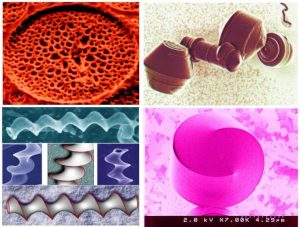 1. Biomimetic Nanochemistry – The paradigm of learning how to transfer Nature’s best materials ideas into the Nanochemistry laboratory, inspired my discovery of ‘morphosynthesis’, a synthetic analogue of morphogenesis, the creation of shapes and patterns in the biological world. My work focused on controlling and understanding, from the nanometer to micron scale, the growth and form of inorganic materials with striking curved shapes and beautiful surface patterns that exhibited ‘natural form’. By natural form, I imply the visual perception of a class of materials with shapes and patterns recognized as being associated with the natural world. These amazing biomimetic constructs were made by template-directed hierarchical-assembly of organic and inorganic molecules and produced for the first time faux diatoms and radiolarian, exotic hollow helicoids and rounded figurines (Nature 1995, Nature 1996, Nature 1997, Nature 1997, ACR 1997).
1. Biomimetic Nanochemistry – The paradigm of learning how to transfer Nature’s best materials ideas into the Nanochemistry laboratory, inspired my discovery of ‘morphosynthesis’, a synthetic analogue of morphogenesis, the creation of shapes and patterns in the biological world. My work focused on controlling and understanding, from the nanometer to micron scale, the growth and form of inorganic materials with striking curved shapes and beautiful surface patterns that exhibited ‘natural form’. By natural form, I imply the visual perception of a class of materials with shapes and patterns recognized as being associated with the natural world. These amazing biomimetic constructs were made by template-directed hierarchical-assembly of organic and inorganic molecules and produced for the first time faux diatoms and radiolarian, exotic hollow helicoids and rounded figurines (Nature 1995, Nature 1996, Nature 1997, Nature 1997, ACR 1997).
 2. Mesoscopic materials – This biomimetic way of thinking about template-directed co-assembly of materials led to my discovery of periodic mesoporous silica in the form of ‘oriented thin films’ formed at liquid-solid and air-solid interfaces (Nature 1995, Nature 1996). These seminal papers inspired a world-wide effort on finding utility for periodic mesoporous silica film in optics, fluidics, microelectronics and sensing, to name a few applications. I also advantageously used template directed co-assembly of meso scale-inorganic materials with compositions beyond the archetype silica.
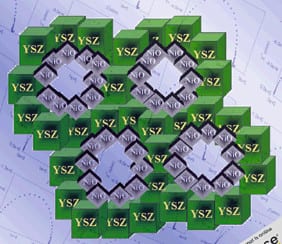
2. Mesoscopic materials – This biomimetic way of thinking about template-directed co-assembly of materials led to my discovery of periodic mesoporous silica in the form of ‘oriented thin films’ formed at liquid-solid and air-solid interfaces (Nature 1995, Nature 1996). These seminal papers inspired a world-wide effort on finding utility for periodic mesoporous silica film in optics, fluidics, microelectronics and sensing, to name a few applications. I also advantageously used template directed co-assembly of meso scale-inorganic materials with compositions beyond the archetype silica. 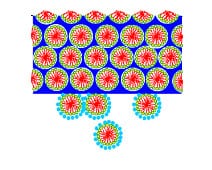 These meso-materials included semiconducting germanium sulfide (Nature 1999), electrochromic nanocrystalline titania (NL 2004), transparent conducting indium tin oxide (AM 2009) and fast ion conducting yttria stabilized zirconia (AFM 2001), which displayed structural features intermediate between the nanoscopic and macroscopic length scales, and compositions that led to many meso-materials useful in today’s energy nanotechnologies.
These meso-materials included semiconducting germanium sulfide (Nature 1999), electrochromic nanocrystalline titania (NL 2004), transparent conducting indium tin oxide (AM 2009) and fast ion conducting yttria stabilized zirconia (AFM 2001), which displayed structural features intermediate between the nanoscopic and macroscopic length scales, and compositions that led to many meso-materials useful in today’s energy nanotechnologies.
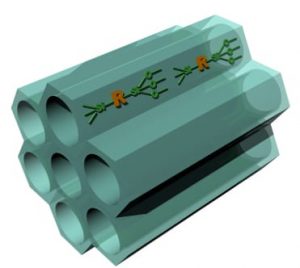 3. Hybrid nanomaterials chemistry – A new class of nanocomposite materials called periodic mesoporous organosilicas (PMOs), were also invented in this phase of my work (Nature 1999). These distinctive hybrid materials contain bridge-bonded organic molecules integrated into the silicate pore walls. The organic moieties include aliphatics, alkenes, aromatics, dendrimers, fullerenes and polyhedral oligomeric silsesquioxanes.
3. Hybrid nanomaterials chemistry – A new class of nanocomposite materials called periodic mesoporous organosilicas (PMOs), were also invented in this phase of my work (Nature 1999). These distinctive hybrid materials contain bridge-bonded organic molecules integrated into the silicate pore walls. The organic moieties include aliphatics, alkenes, aromatics, dendrimers, fullerenes and polyhedral oligomeric silsesquioxanes. 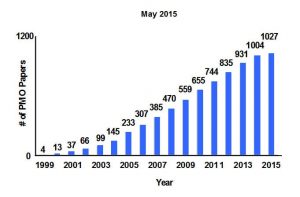 Today, having undergone extraordinary growth across the globe and across the borders of the science disciplines, PMOs deliver properties that transcend the sum of their inorganic and organic components and are finding widespread application as interlayer dielectrics in microelectronic packaging, chromatography stationary phases, chiral catalysis, dental implants and drug delivery vehicles (Science 2003, Science 2004, ACR 2005).
Today, having undergone extraordinary growth across the globe and across the borders of the science disciplines, PMOs deliver properties that transcend the sum of their inorganic and organic components and are finding widespread application as interlayer dielectrics in microelectronic packaging, chromatography stationary phases, chiral catalysis, dental implants and drug delivery vehicles (Science 2003, Science 2004, ACR 2005).
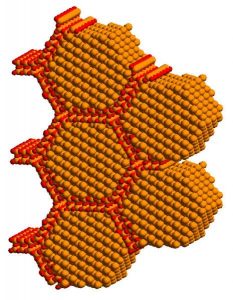 4. Host-guest inclusion chemistry – Using chemical vapor deposition and metal organic vapor deposition within the spatial confines of nanoporous hosts, I discovered how to control the nucleation and growth, stabilization and protection of size- and shape-controlled quantum-confined semiconductor nanomaterials, exemplified by nanometer dimension Si, Ge, Ag, AgCl, CdS, SnS2, MoO3 and WO3, (ACR 1992) This genre of research inspired subsequent work on ligand-stabilized colloidal nanocrystals that underpin some of today’s most promising nanotechnologies including solar cells and batteries, super-capacitors and fuel cells, medical diagnostics, imaging and theranostics.
4. Host-guest inclusion chemistry – Using chemical vapor deposition and metal organic vapor deposition within the spatial confines of nanoporous hosts, I discovered how to control the nucleation and growth, stabilization and protection of size- and shape-controlled quantum-confined semiconductor nanomaterials, exemplified by nanometer dimension Si, Ge, Ag, AgCl, CdS, SnS2, MoO3 and WO3, (ACR 1992) This genre of research inspired subsequent work on ligand-stabilized colloidal nanocrystals that underpin some of today’s most promising nanotechnologies including solar cells and batteries, super-capacitors and fuel cells, medical diagnostics, imaging and theranostics.
5. Photonic crystal materials – I discovered how to employ self-assembly to synthesize the world’s first 3D silicon photonic crystal with an omni-directional photonic bandgap operating at optical telecom wavelengths (Nature 2000). 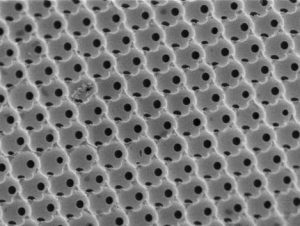 This genre of research inspired me to synthesize photonic crystals with a wide range of compositions that could display the full gamut of ‘color from structure’ as found in the natural world. I also discovered how the ‘slow light’ attributes of these photonic crystals could be used to amplify light absorption, which inspired me to use them to enhance the efficiency of silicon and titania solar cells and titania photocatalysts (NL 2011, 2013, AM 2007, 2009). I also showed how to incorporate nanoscale planar defects into photonic crystals and how to implement them as a new class of chemical and biological color sensors (AM 2006).
This genre of research inspired me to synthesize photonic crystals with a wide range of compositions that could display the full gamut of ‘color from structure’ as found in the natural world. I also discovered how the ‘slow light’ attributes of these photonic crystals could be used to amplify light absorption, which inspired me to use them to enhance the efficiency of silicon and titania solar cells and titania photocatalysts (NL 2011, 2013, AM 2007, 2009). I also showed how to incorporate nanoscale planar defects into photonic crystals and how to implement them as a new class of chemical and biological color sensors (AM 2006).
 6. Smart mirrors – In a flurry of trendsetting papers I showed how to synthesize alternating composition multi-layers made from a wide range of nanomaterials comprised of main group and transition metal oxides, zeolites, mesoporous materials and clays. These self-assembled Bragg mirrors provided high porosity and large surface area, ion-exchange and molecule size discriminating properties to the constituent layers. This enabled active tuning of the structural color of reflected or transmitted light through chemically and physically induced changes in the thicknesses and/or refractive indices of the constituent layers and led to the development of a new class of colorimetric sensors and anti-bacteria patches with controlled release and detection capabilities. I also showed that Bragg mirrors made from transparent and conducting antimony and indium tin oxides, enabled the development of improved- performance organic light-emitting diodes, grey scale electrochromics and a new genre of solid state dye and polymer lasers (CSR 2013).
6. Smart mirrors – In a flurry of trendsetting papers I showed how to synthesize alternating composition multi-layers made from a wide range of nanomaterials comprised of main group and transition metal oxides, zeolites, mesoporous materials and clays. These self-assembled Bragg mirrors provided high porosity and large surface area, ion-exchange and molecule size discriminating properties to the constituent layers. This enabled active tuning of the structural color of reflected or transmitted light through chemically and physically induced changes in the thicknesses and/or refractive indices of the constituent layers and led to the development of a new class of colorimetric sensors and anti-bacteria patches with controlled release and detection capabilities. I also showed that Bragg mirrors made from transparent and conducting antimony and indium tin oxides, enabled the development of improved- performance organic light-emitting diodes, grey scale electrochromics and a new genre of solid state dye and polymer lasers (CSR 2013).
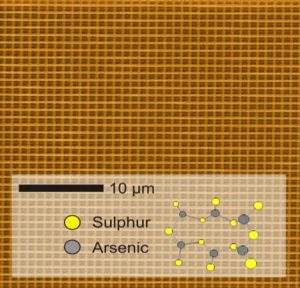 7. Multi-photon direct laser written (DLW) photonic bandgap nanomaterials – In collaboration with colleagues at the Karlsruhe Institute of Technology, I used this nanofabrication method to invert a DLW polymer template in silica by atomic layer deposition. This enabled a subsequent inversion in silicon by disilane chemical vapor deposition, creating thereby a silicon replica of the original polymer template (Nature Materials 2006). Silicon photonic bandgap nanomaterials created by this inventive ‘double inversion’ method facilitate the development of silicon-based all-optical devices, circuits and chips with utility in optical telecommunication and computer systems. I spearheaded a creative extension of this work with single-step DLW in a high refractive index ‘inorganic’ photo-resist, arsenic sesquisulphide, As2S3. This opened the door to a large variety of new photonic bandgap materials and architectures that can be made by DLW without inversion of a sacrificial polymer template (ChemMater 2008).
7. Multi-photon direct laser written (DLW) photonic bandgap nanomaterials – In collaboration with colleagues at the Karlsruhe Institute of Technology, I used this nanofabrication method to invert a DLW polymer template in silica by atomic layer deposition. This enabled a subsequent inversion in silicon by disilane chemical vapor deposition, creating thereby a silicon replica of the original polymer template (Nature Materials 2006). Silicon photonic bandgap nanomaterials created by this inventive ‘double inversion’ method facilitate the development of silicon-based all-optical devices, circuits and chips with utility in optical telecommunication and computer systems. I spearheaded a creative extension of this work with single-step DLW in a high refractive index ‘inorganic’ photo-resist, arsenic sesquisulphide, As2S3. This opened the door to a large variety of new photonic bandgap materials and architectures that can be made by DLW without inversion of a sacrificial polymer template (ChemMater 2008).
 8.Photonic crystal Nanochemistry – This research on photonic crystal nanomaterials enabled me to invent actively-tuned ‘photonic color’ systems (Nature Materials 2006, Nature Photonics 2007) now commercialized by Opalux, a spin-off company that I co-founded in 2006. These technology platforms include full color displays, authentication devices for anti-counterfeiting, color sensors for food and water quality control and pathogen detection (www.opalux.com).
8.Photonic crystal Nanochemistry – This research on photonic crystal nanomaterials enabled me to invent actively-tuned ‘photonic color’ systems (Nature Materials 2006, Nature Photonics 2007) now commercialized by Opalux, a spin-off company that I co-founded in 2006. These technology platforms include full color displays, authentication devices for anti-counterfeiting, color sensors for food and water quality control and pathogen detection (www.opalux.com).
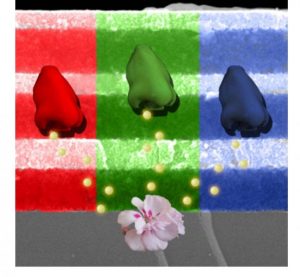 9. Seeing the light – One of the hallmarks of my research is the creative exploitation of the unique properties of regular arrangements of nanopores with dimensions that traverse nanometers to microns. For example, my research on periodic macroporous materials, which I aptly calls ‘light-scale’ materials, has been focused on electrically, thermally, mechanically, and chemically tuned ‘color from structure’. This revolutionary concept forms the basis of a new ‘photonic color’ nanotechnology being developed by Opalux who are introducing three unique manifestations of this nanotechnology to the market. P-Ink is a flexible, electronic paper-like material offering a full spectrum of electrically-tunable, reflective colors. Being bi-stable and power-efficient, it is one of three competitive technologies vying to add color to black-and-white electronic book readers such as Kindle and Kobo. P-Nose is an artificial nose comprised of a simple, cost-effective pixilated array of surface-functionalized nanoporous materials that enable discrimination of different analytes, such as molecules comprising the unique identifiers of different bacteria. Think of the possibilities for medical diagnostics, and food and water quality-control. Elast-Ink is a touch-sensitive material that responds to mechanical pressure while offering exceptional resolution and customizability. It is poised to answer global demand for effective authentication-technology, serving, for example, the pharmaceutical and banknote-printing industries.
9. Seeing the light – One of the hallmarks of my research is the creative exploitation of the unique properties of regular arrangements of nanopores with dimensions that traverse nanometers to microns. For example, my research on periodic macroporous materials, which I aptly calls ‘light-scale’ materials, has been focused on electrically, thermally, mechanically, and chemically tuned ‘color from structure’. This revolutionary concept forms the basis of a new ‘photonic color’ nanotechnology being developed by Opalux who are introducing three unique manifestations of this nanotechnology to the market. P-Ink is a flexible, electronic paper-like material offering a full spectrum of electrically-tunable, reflective colors. Being bi-stable and power-efficient, it is one of three competitive technologies vying to add color to black-and-white electronic book readers such as Kindle and Kobo. P-Nose is an artificial nose comprised of a simple, cost-effective pixilated array of surface-functionalized nanoporous materials that enable discrimination of different analytes, such as molecules comprising the unique identifiers of different bacteria. Think of the possibilities for medical diagnostics, and food and water quality-control. Elast-Ink is a touch-sensitive material that responds to mechanical pressure while offering exceptional resolution and customizability. It is poised to answer global demand for effective authentication-technology, serving, for example, the pharmaceutical and banknote-printing industries.
It is worth pointing out that the P-Ink photonic color technology developed by Oplaux was recognized by the Technical Development Materials Award in the USA in 2011, which identifies the most innovative and significant technical achievement in the field of materials development (http://www.idtechex.com/printed-electronics-usa-11/awards.asp). Opalux follows in the footsteps of many previous illustrious industry winners of this award in the US, Europe and Asia. In 2013, Opalux P-Ink Photonic Technology received the Global Innovation Award for its potential impact on the specialty colour displays industrial sector (http://techconnectworld.com/World2013/participate/innovation/innovation_awards.html). Opalux Opal-Print Technology was runner-up in the 2013 Excellence in Tax Stamps Awards for best new innovation in anti-counterfeiting, anti-diversion, document security, brand protection and holography technologies (http://www.taxstampnews.com/awards).
 10. Clever new Nanochemistry twists – I was amongst the first few scientists to demonstrate chemically-powered ‘nano locomotion’. My work was based on chemical control of the motion of barcode nanorod motors, whose power is obtained from the decomposition of hydrogen peroxide into water and oxygen localized at the catalytic segment of the nanorod (ChemComm 2005, AM 2005). First experiments were aimed at nanorod rotors and motors and understanding the origin and control of their motion and speed. Subsequently I was the first to show how to make them flexible by integrating polymer hinges between the segments of the nanorod (Nature Nanotechnology 2007).
10. Clever new Nanochemistry twists – I was amongst the first few scientists to demonstrate chemically-powered ‘nano locomotion’. My work was based on chemical control of the motion of barcode nanorod motors, whose power is obtained from the decomposition of hydrogen peroxide into water and oxygen localized at the catalytic segment of the nanorod (ChemComm 2005, AM 2005). First experiments were aimed at nanorod rotors and motors and understanding the origin and control of their motion and speed. Subsequently I was the first to show how to make them flexible by integrating polymer hinges between the segments of the nanorod (Nature Nanotechnology 2007).  These papers have inspired a veritable ‘nanomotor industry’. Activity in this field is now burgeoning around the world with envisioned nanomachine applications that include the removal of pollutants from water and as drug-carrying and drug delivery vehicles for targeted cancer therapy. Another innovation to emerge from my research involved the discovery of ultrathin inorganic nanowires (ACIE 2008), which are characterized by unprecedented small < 2 nm diameters. These amazingly thin nanowires look, grow and behave like organic polymers (JACS 2012, 2010, NL 2009, AM 2009). This work inspired a flurry of activity around the globe to explore the composition space and structure, properties, and functionality of these uniquely-thin one-dimensional constructs. This work raised an important question about how to expand and enrich the myriad applications enjoyed by organic polymers into the completely uncharted territory of ultrathin inorganic nanowires. The opportunities appear to be boundless!
These papers have inspired a veritable ‘nanomotor industry’. Activity in this field is now burgeoning around the world with envisioned nanomachine applications that include the removal of pollutants from water and as drug-carrying and drug delivery vehicles for targeted cancer therapy. Another innovation to emerge from my research involved the discovery of ultrathin inorganic nanowires (ACIE 2008), which are characterized by unprecedented small < 2 nm diameters. These amazingly thin nanowires look, grow and behave like organic polymers (JACS 2012, 2010, NL 2009, AM 2009). This work inspired a flurry of activity around the globe to explore the composition space and structure, properties, and functionality of these uniquely-thin one-dimensional constructs. This work raised an important question about how to expand and enrich the myriad applications enjoyed by organic polymers into the completely uncharted territory of ultrathin inorganic nanowires. The opportunities appear to be boundless!
 Lately, I have developed a passion for a greener kind of Nanochemistry and figured out how to separate poly-dispersions of quantum-confined silicon nanocrystals into mono-dispersed colloidally-stable fractions with tailored organic surfaces (JACS 2011). Incredibly, for the archetype semiconductor silicon, this feat was the first of its kind since the discovery of silicon nanocrystals more than thirty years ago. The brightly colored visible to near infrared photoluminescence of these size-separated silicon nanocrystals enabled determination of their size-dependent absolute quantum yields, (NL 2012). These photoluminescence quantum yields were found to be surprisingly high and as a result are targeted for a range of ‘green’ nanotechnologies that include multi-color light-emitting diodes and biomedical diagnostics, and therapeutics and imaging for detecting and targeting tumors. I believe ‘green Nanochemistry’ founded on benign nanocrystalline silicon will help alleviate the fear of cytotoxicity that pervades the use of heavy metal chalcogenide and pnictide nanomaterials currently favored for advanced materials and biomedical nanotechnologies (NL 2011, 2012, 2013, AM 2012, Small 2012).
Lately, I have developed a passion for a greener kind of Nanochemistry and figured out how to separate poly-dispersions of quantum-confined silicon nanocrystals into mono-dispersed colloidally-stable fractions with tailored organic surfaces (JACS 2011). Incredibly, for the archetype semiconductor silicon, this feat was the first of its kind since the discovery of silicon nanocrystals more than thirty years ago. The brightly colored visible to near infrared photoluminescence of these size-separated silicon nanocrystals enabled determination of their size-dependent absolute quantum yields, (NL 2012). These photoluminescence quantum yields were found to be surprisingly high and as a result are targeted for a range of ‘green’ nanotechnologies that include multi-color light-emitting diodes and biomedical diagnostics, and therapeutics and imaging for detecting and targeting tumors. I believe ‘green Nanochemistry’ founded on benign nanocrystalline silicon will help alleviate the fear of cytotoxicity that pervades the use of heavy metal chalcogenide and pnictide nanomaterials currently favored for advanced materials and biomedical nanotechnologies (NL 2011, 2012, 2013, AM 2012, Small 2012).
 11. Nanochemistry education – Another aspect of my work worth mentioning involves education. My textbooks ‘Concepts in Nanochemistry’ and ‘Nanochemistry: A Chemical Approach to Nanomaterials’, co-authored with former students Andre Arseault and Ludovico Cademartiri, are globally acclaimed as the gold standard reference works for teaching Nanochemistry to both undergraduate and graduate students.
11. Nanochemistry education – Another aspect of my work worth mentioning involves education. My textbooks ‘Concepts in Nanochemistry’ and ‘Nanochemistry: A Chemical Approach to Nanomaterials’, co-authored with former students Andre Arseault and Ludovico Cademartiri, are globally acclaimed as the gold standard reference works for teaching Nanochemistry to both undergraduate and graduate students.
My out-reach efforts through insightful and engaging lectures and monthly opinion editorials in Wiley materials journals (http://www.materialsviews.com/author/gozin/) aim to inform scientists and laypersons alike on pressing issues affecting the future of all of us. Hopefully, these inspiring, thought-provoking perspectives offer viable ways of improving the state of the world.
 12. ArtNanoInnovations – In 2011 in collaboration with artist Todd Siler, I co-founded ArtNanoInnovations the mission being to explore the realization of nature-inspired innovations in nanoscience and nanotechnology, which aim to benefit humankind by meeting our global challenges. This work entails (connecting and transforming) the myriad forms of nanometer scale science through multimedia artworks and aesthetic experiences that connect us with Nature’s creations, which we can build on in creating a sustainable future, www.artnanoinnovations.com.
12. ArtNanoInnovations – In 2011 in collaboration with artist Todd Siler, I co-founded ArtNanoInnovations the mission being to explore the realization of nature-inspired innovations in nanoscience and nanotechnology, which aim to benefit humankind by meeting our global challenges. This work entails (connecting and transforming) the myriad forms of nanometer scale science through multimedia artworks and aesthetic experiences that connect us with Nature’s creations, which we can build on in creating a sustainable future, www.artnanoinnovations.com.
What is next in Nanochemistry?
 The genre of Nanochemistry research outlined above has provided the foundation for the most recent phase of my research on new nanomaterials for enabling a global energy revolution. The vision is based upon the discovery of nanostructured photo-catalysts capable of both capturing and converting gaseous CO2 into solar fuels that will replace their fossil fuel counterparts, ameliorate climate change and power our planet for the foreseeable future. It is clear that in order to address these crucial issues, new chemistry that enables advance energy materials and technologies must be developed (EES 2015, AM 2015).
The genre of Nanochemistry research outlined above has provided the foundation for the most recent phase of my research on new nanomaterials for enabling a global energy revolution. The vision is based upon the discovery of nanostructured photo-catalysts capable of both capturing and converting gaseous CO2 into solar fuels that will replace their fossil fuel counterparts, ameliorate climate change and power our planet for the foreseeable future. It is clear that in order to address these crucial issues, new chemistry that enables advance energy materials and technologies must be developed (EES 2015, AM 2015).
This is where Nanochemistry research and very recent exciting developments in my group come into the picture. My group is a member of a University of Toronto multi-disciplinary solar fuels team of experimental and theoretical materials chemists and engineers, working together to build a materials technology that can simultaneously harness abundant solar energy, capture and reduce gaseous carbon dioxide into fuels and chemical feed stocks, simultaneously addressing issues of energy security and climate change.
The inventiveness and practicality of our early work in this field can be showcased with two of our recent discoveries. The first involves, the rational design of a single-component photocatalyst for the efficient gas-phase CO2 reduction using both UV and visible light (AS 2014). The second concerns the efficient photomethanation of gaseous CO2 on ultra-black silicon nanowire catalyst supports with visible and near-infrared photons, a step towards broadband solar fuels reactors (AS 2014). In this context it is noteworthy that even a modest target conversion rate of 10 mole CO2 per hour per gram of photocatalyst would translate into a conversion rate of 1 Gt CO2 per year per ton of photocatalyst, which configured as solar fuels panels could be incorporated into solar fuels chimneys built on land or floating on water to minimize usage of property. With an earth abundant, non-toxic, cost effective, scalable catalyst integrated with existing chemical and petrochemical industrial infrastructure, one can begin to appreciate that CO2 conversion rates of this magnitude can provide a potentially practical, economical and sustainable alternative to burning and depleting fossil fuel reserves.
My vision for an energy transition from one based on unsustainable fossil fuels to a sustainable solar fuels energy technology founded on capturing and utilizing CO2 – from both thin air and more concentrated localized sources – is compatible with existing CO2 emitting industries around the world. To achieve this vision of a carbon-neutral air-to-fuel carbon-cycle technology my group is currently developing compact, tandem, concentrated solar powered photochemical reactors for efficiently splitting gaseous water first into H2 and then using the H2 to reduce gaseous CO2 to fuels and chemicals. I believe the time it should take to convert solar fuels laboratory-scale science to a global technology could be short enough to circumvent the predicted adverse consequences of greenhouse gas climate change, enabling a timely energy transition from fossil fuels to solar fuels.
Nanochemistry final thoughts
It is tremendously satisfying that my contributions to fundamental research laid out the essence of a chemical approach to nanomaterials, defining the conceptual foundations and securing credibility for a novel scientific discipline. I am thrilled by the way that this new discipline of Nanochemistry has developed and matured over the last 20 years and continues to serve as an integral driver of further developments in many tangential scientific undertakings which we now count upon to catalyze scientific, industrial and economic advancement. Of course none of my discoveries in the field of Nanochemistry would have been possible without the incredible contributions of a large cadre of highly creative coworkers many of which have gone on to academic positions in top notch universities, scientific positions in national laboratories and chemical industries, and founded spin-off companies around the world. I am also indebted for the tremendous support and encouragement of a large number of University of Toronto colleagues, provincial and federal funding agencies, national and international collaborators, industrial partners and of course my wife and best friend, Linda Ozin. The RSC Centenary Award in recognition of my career’s work in this exciting and developing discipline is deeply treasured.
Acknowledgments
G.A.O. is Government of Canada Tier 1 Canada Research Chair in Materials Chemistry and Nanochemistry. Strong and sustained financial support from the Ontario Ministry of Research and Innovation (MRI), the Ontario Ministry of Economic Development and Innovation (MEDI), the Natural Sciences and Engineering Council of Canada (NSERC), the Connaught Innovation Fund and the University of Toronto is deeply appreciated. The creative contributions of the many faculty and students to the work described in this Perspective are deeply treasured.