By Quang Trung Truong and Byung Hee Hong
Graphene, a single layer of carbon atoms arranged into honeycomb lattice, a so-called “wonder materials” with its fantastic properties, has continuously attracted researchers’ attention since 2004 due to its high carrier mobility, superior electrical conductivity, high thermal conductivity, high Young’s modulus, and high specific surface area. These unique properties of graphene are being exploited for many potential applications in various fields such as high performance polymer nanocomposites, sensors, energy storage & conversion (supercapacitors, batteries, LEDs, solar cells), field-effect transistors, conductive inks for printed electronics, transparent conducting films, catalysis, water purification/ desalination, biomedical applications (biosensors, bio-devices), drug/gene delivery and cancer therapy – the list is almost endless.
Despite these potential applications, and all of the recent research progress in graphene, widespread applications in day-to-day life have yet to appear. This is for a variety of reasons, but the main issue is how to mass produce graphene in a simple process at a reasonable cost.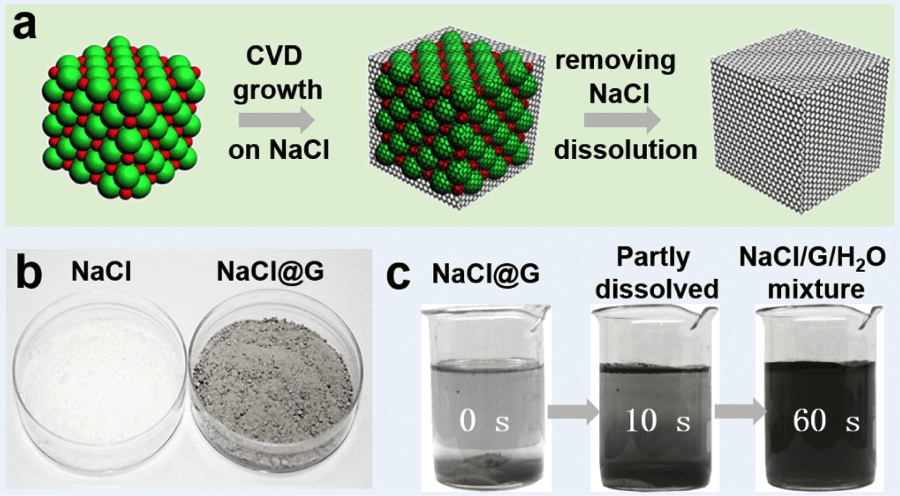
Currently, various synthetic methods for graphene have been explored, mainly including mechanical and chemical exfoliation of graphite, sublimation of epitaxial SiC, and chemical vapor deposition (CVD) using hydrocarbons. Among these, CVD growth of graphene on metallic or ceramic substrates using different hydrocarbon sources has been the most promising method for obtaining high-quality graphene.
However, when graphene is grown on a metallic or ceramic substrate, a wet-chemical transfer process is inevitably involved to remove the substrates, which leads to damage to or even consumption of the substrates. The remaining waste requires recycling, will increases the total cost of graphene produced in this manner.
Furthermore, in the etching transfer of metal substrates, in which a polymer supporting film (poly methyl methacrylate in most cases) is coated onto the graphene, there is always the risk of additional contamination.
Therefore, a novel, low-cost substrate, and an easy and efficient approach for dissolving the substrate after the graphene growth, is required to obtain pure graphene without any impurities.
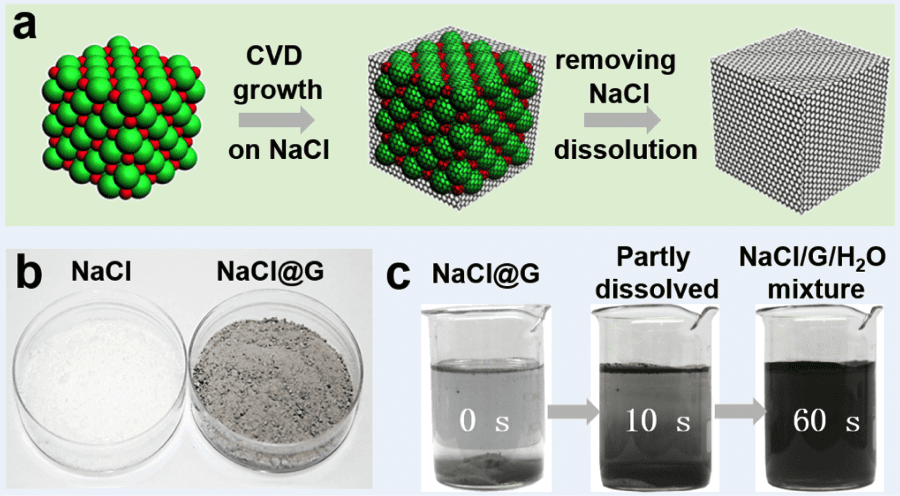
Professor Zhongfan Liu and co-workers from College of Chemistry and Molecular Engineering, Peking University have now investigated such a substrate, where micron-size sodium chloride crystals have been used as a water soluble, recyclable substrate for CVD growth of few layers of graphene.
In their CVD process, the six facets the NaCl crystals were considered to serve as CVD growth fronts for graphene synthesis. The commercial NaCl salt (average size: 300 μm) was firstly recrystallized to obtain NaCl powder with an average crystal size of 10 μm before being introduced into the low-temperature zone of a two-zones furnace.
The high-temperature zone was heated to 850 °C to promote the thermal cracking of the carbon source (ethylene) and graphene was synthesized over NaCl powder in the low-temperature zone (700 °C). The low-temperature zone was maintained at this temperature, which is much lower than the melting point of NaCl (801 °C), in order to prevent melting of the NaCl powder. The graphene growth on NaCl was completed in 2 h and the furnace was then cooled down to room temperature. Free-standing graphene powder was then obtained after dissolving the core NaCl in water.
They also found that when large NaCl crystals (>300 μm) are used, no graphene is grown, which means that the small crystal size plays a vital role in the graphene growth mechanism.
The grown graphene crystals present cubic shapes ranging from 1 to 30 μm in length, retaining the original shapes and sizes of NaCl crystals. The cuboidal graphene was characterized using different analysis techniques such as SEM, TEM, Raman, AFM images and they found that graphene with less than 5 layers accounted for 70 % of the total synthesized.
The outstanding results of this synthetic method pave a new path for facile and low-cost preparation of graphene with few layers in large quantities, and bring out a bright future for practical application of graphene, especially in areas such as polymer nanocomposites, energy storage (supercapacitors, batteries), conductive inks for printed electronics, catalysis and water purification.
 Quang Trung Truong is a senior research engineer at the R&D department of Graphene Square Inc, a spin-off company from Seoul National University, founded by Professor Byung Hee Hong. He holds a PhD degree in Chemical Engineering from Chonbuk National University, Korea where he studied graphene synthesis from natural graphite and their polymer nanocomposites. He is currently working on CVD synthesis and transfer of large area graphene and 2D materials for electronics applications.
Quang Trung Truong is a senior research engineer at the R&D department of Graphene Square Inc, a spin-off company from Seoul National University, founded by Professor Byung Hee Hong. He holds a PhD degree in Chemical Engineering from Chonbuk National University, Korea where he studied graphene synthesis from natural graphite and their polymer nanocomposites. He is currently working on CVD synthesis and transfer of large area graphene and 2D materials for electronics applications.
 Byung Hee Hong(b. 1971) received BS (1998), MS (2000) and PhD (2002) degrees in chemistry from POSTECH in Korea. After spending three and a half years as a postdoctoral researcher at Columbia University (Advisor: Philip Kim), he joined the Department of Chemistry, Sungkyunkwan University (SKKU) as an Assistant Professor in 2007. He is now an Associate Professor in the Department of Chemistry at Seoul National Univ. Prof. Hong pioneered the large-scale synthesis of graphene by CVD, which triggered chemical research studies toward the practical applications of graphene
Byung Hee Hong(b. 1971) received BS (1998), MS (2000) and PhD (2002) degrees in chemistry from POSTECH in Korea. After spending three and a half years as a postdoctoral researcher at Columbia University (Advisor: Philip Kim), he joined the Department of Chemistry, Sungkyunkwan University (SKKU) as an Assistant Professor in 2007. He is now an Associate Professor in the Department of Chemistry at Seoul National Univ. Prof. Hong pioneered the large-scale synthesis of graphene by CVD, which triggered chemical research studies toward the practical applications of graphene