 The challenging task of designing organic crystals with targeted linear and nonlinear optical properties has just been made a whole lot easier.
The challenging task of designing organic crystals with targeted linear and nonlinear optical properties has just been made a whole lot easier.
T. Seidler, B. Champagne, and co-workers have taken advantage of a multi-disciplinary approach, combining the synthesis of the active molecules, the characterization of their properties, the crystallization and the determination of the crystal structure and properties, and numerical simulations based on theoretical models.
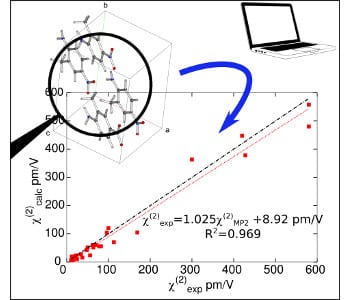
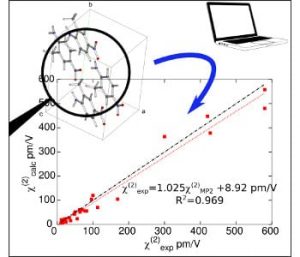
Agreement between the calculations and measurements is shown to be excellent, demonstrating the ability of a multi-scale numerical simulation approach to predict and interpret the linear (refractive indices) and nonlinear (second-order nonlinear optical susceptibilities) optical properties for a broad range of organic crystals, solely from the knowledge of their crystal structure.
This rapid screening method combines quantum chemical calculations of the molecular properties with classical electrostatics to describe the crystal environment effects.