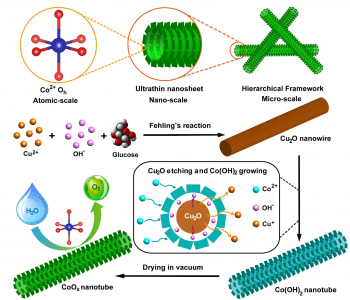
Taking a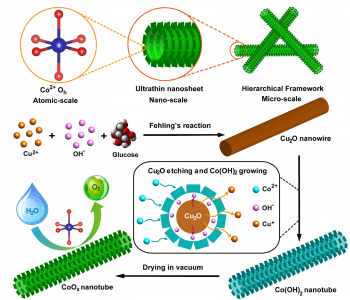 leaf out of nature’s design book, researchers at Fudan University and Soochow University, China, have developed hierarchical CoOx nanosheet/nanotube structures to improve the efficiency of oxygen evolution reaction (OER) catalysts. The novel hybrid structure leads to an ultrahigh surface area of 371 m2 g−1, yielding the most efficient cobalt-based OER catalysts reported thus far.
leaf out of nature’s design book, researchers at Fudan University and Soochow University, China, have developed hierarchical CoOx nanosheet/nanotube structures to improve the efficiency of oxygen evolution reaction (OER) catalysts. The novel hybrid structure leads to an ultrahigh surface area of 371 m2 g−1, yielding the most efficient cobalt-based OER catalysts reported thus far.
Inspiration comes from the natural plant structure, having flat leaves with large surface area overlapping as little as possible, and, under the surface, arrays of hollow tubes for transportation of water and nutrients for the various biofunctions performed in the leaves. This structure is mimicked in the novel nanosheet/nanotube structure of this OER catalyst, which consists of CoOx nanotubes, with ultra-thin nanosheets arranged on the surface. The nanosheets result in an extremely high surface area over which the reaction can occur, and the hollow nanotubes supporting the nanosheets allow for excellent electrical transport during the reaction. Synthesis of the catalyst is achieved by first growing Cu2O nanowire templates, and then reacting with Na2S2O3 and CoCl2. The Na2S2O3 etches away the nanowire, releasing OH─ ions, which react with Co2+ to precipitate CoOx nanosheets around the nanowires. Upon drying, either in air or in vacuum, the nanotubes are further assembled into a tangled framework, highly porous and efficient in for conduction between the individual nanotubes. In addition to this enhancement of the physical structure, the chemical environment of the cobalt ions is also optimized, with a high proportion of Co2+ ions in the efficient octahedral configuration.
The hybrid structure shows a low reaction onset potential of 1.46 V, comparable to IrO2 nanoparticles, a standard OER catalyst. This value is significantly lower than that of other cobalt-based catalysts. The vacuum-dried catalyst shows a higher current density compared to the air-dried structure, with performance remaining at 65% after 100 minutes, compared to 56% for the air-dried. The performance of the vacuum-dried hybrid structure is the highest reported thus far for cobalt-based OER catalysts.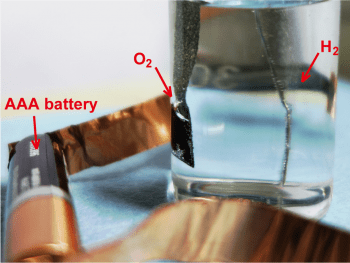
Electrochemical water splitting reactions are one of the most promising methods for hydrogen generation towards a cleaner energy source. A crucial step in water splitting is the OER reaction, which is a multistep process requiring four electrons, and thus is kinetically sluggish. Due to their high activity, stability, abundance, and lack of environmental impact, cobalt-based catalysts have been suggested for applications as the OER catalyst in water splitting reactions. The hybrid structured cobalt-based catalyst described here represents a significant step forward in overcoming the challenges of simultaneous structure control over multi-scale levels, that have thus far hindered practical application of cobalt-based catalysts fot water splitting. The potential for use of this catalyst as the anode in a water splitting cell is demonstrated by successful and continuous water splitting using a standard 1.5 V AAA battery, using a platinum cathode.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.