Billions of years ago, Earth was a vastly different planet characterized by a harsh environment that could not support life. Volcanic eruptions, lightning storms, and the constant bombardment of meteorites and comets shaped its atmosphere and surface.
These tumultuous conditions were instrumental in creating organic molecules, such as amino acids, nucleotides, and simple sugars, that would eventually lead to life as we know it. But how exactly this transformation occurred remains one of the most enduring mysteries in scientific research.
In a recent study published in Small Methods, a team of scientists presents an intriguing hypothesis. They propose that “non-biological” organic molecules — those that are carbon-based but not typically used by biological systems today — may have played a pivotal role in helping primitive chemical systems evolve into their current, complex forms.
“No one really knows exactly what happened on early Earth or what chemicals or reactions were present,” explained Tony Z. Jia, specially appointed associate professor at the Earth-Life Science Institute, Tokyo Institute of Technology and one of the study’s lead authors, in an email.
“While it’s impossible to know exactly without a time machine, we do our best to replicate plausible conditions in the lab in the hopes of understanding how early Earth chemistry resulted in the origin of life,” he added.
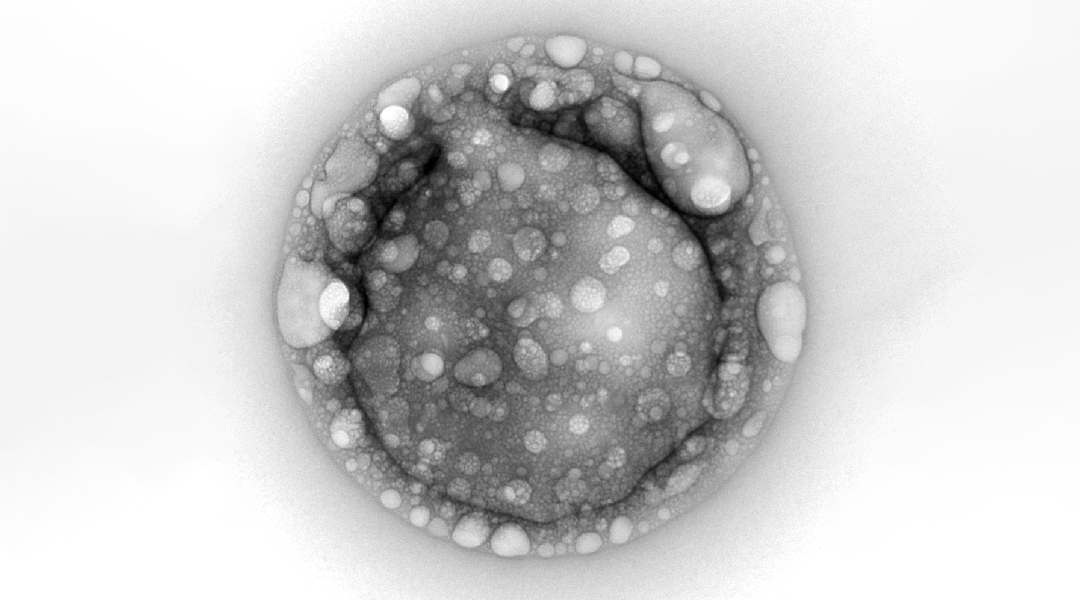
Polyester microdroplets — protocell models?
Modern life is water-dependent, and it is widely believed that life originated in ancient aqueous environments. To better understand this hypothesis, the researchers in the present study focused on α-hydroxy acids, molecules similar in structure to α-amino acids, which could have been present on the early Earth, possibly formed through electrical sparks, hydrothermal reactions, ultraviolet photochemistry, or even delivered by meteorites.
What is interesting is that in solutions that simulate primitive aqueous environments on the early Earth, α-hydroxy acids form gel-like polyesters, which assemble into microdroplets following dehydration and rehydration. The team suggests that if such droplets did exist, they might have also absorbed salts — essential components of living systems.
“Early oceans were suggested to have a much higher level of salt compared to the salinity today,” added Chen Chen, special postdoctoral researcher at RIKEN (previously a research scientist at the Earth-Life Science Institute, Tokyo Institute of Technology, where this work was completed), and co-lead author of the study. “In particular, many kinds of salt ions still play key roles in regulating diverse, healthy biochemical activities in modern life.”
The presence of salts could have influenced the structure of these microdroplets by segregating or concentrating essential biomolecules, like RNA. As such, they could have served as early protocells — simplified versions of primitive cells.
“Polyester microdroplets were first proposed as protocell models by researchers at ELSI a few years ago, and since then we’ve made quite a bit of progress in understanding their composition, structure, and function,” said Jia. “In a previous study, we observed that adding salt caused the droplets to coalesce, and always wondered why.”
The scientists speculate that these primitive microdroplets may have possessed primitive features associated with living systems, such as the ability to carry out chemical reactions and maintain a certain level of internal organization.
“Recently, researchers have been studying membrane-less compartments as an antecedent to membrane-based compartments,” explained Sudha Rajamani, an astrobiologist at the Indian Institute of Science Education and Research (IISER), Pune, who was not involved in the study. “Few studies have dealt with characterizing droplets made of prebiotically plausible — non-synthetic — precursors.”
Whether the polyester microdroplets led directly to the first cells or if they were instead an intermediate state remains unknown. But their propensity to form and their ability to uptake analytes, like salts, could help answer important questions.
“The formation of biopolymers, such as RNA and peptides, from their monomers and the formation of protocellular compartments performing rudimentary cellular functions, are two of the main unsolved problems for the investigation of the origin of life,” said Tommaso Fraccia, an assistant professor of pharmacological and biomolecular sciences at the University of Milano and a soft-matter physicist with expertise in liquid-liquid phase separations who was not involved in the study.
“This study outlines how a system that, starting from small prebiotically plausible molecules (α-hydroxy acids), can efficiently produce polymers by simple processes (drying and heating), which in turn assemble in membraneless compartments upon rehydration,” he added. “The simplicity of the system composition and of the involved processes are very important to propose robust solutions to the polymerization and compartmentalization problems in prebiotic scenarios.”
Studying salt uptake with new techniques
The prebiotic chemical environment was complex and chaotic. “We therefore wondered how the coexisting salts could have affected protocellular dynamics, such as stability and coalescence, in the primitive environment,” said Chen.
“Salt uptake by polyester microdroplets may not directly contribute to the emergence of life, but it would have some implications for the stability and functionality of the microdroplets as potential protocells,” he continued. “Salts can act as catalysts or modulators of certain chemical reactions relevant to the origin of life and can also affect the molecular interactions that occur within and around the polyester microdroplets.”
To recreate these conditions, the research team subjected a series of α-hydroxy acids to dehydration, resulting in the formation of polyesters which were then screened for their ability to assemble into microdroplets.
“With tons of unregulated reactions taking place amongst a huge number of chemicals all mixed together, the chemical composition may have been different depending on [the] environment,” said Jia. “In fact, it’s even possible that the early Earth chemistry looked quite different to what we see in biology today, which is why we need to understand more about how polyesters and other molecules not overly present in modern biology could have contributed to the origin of life.”
Among the α-hydroxy acids they examined, DL-3-phenyllactic acid showed a strong tendency to form droplets and was chosen as a model for their studies. Since electrostatic interactions between salt molecules and the polyester polymers would likely govern the uptake of salt into the microdroplets, the researchers created three variants of α-hydroxy acids: neutral, basic, and acidic.
The scientists then incubated the different polyester microdroplets in aqueous solutions consisting of different concentrations of chloride salts (such as NaCl, KCl, MgCl2, and CaCl2) that may have been abundant in early oceans.
To analyze the amount of salt cations absorbed by the polyester microdroplets, they employed an advanced analytical technique called inductively coupled plasma mass spectrometry (ICP-MS), an analytical technique that can be used to measure elements at trace levels in biological fluids. This was done at the Pheasant Memorial Lab at the Institute of Planetary Materials at Okayama University, where scientists typically use this technique to analyze extraterrestrial samples, such as those from meteorites like Ryugu.
Jia emphasized the significance of applying such cutting-edge techniques from adjacent fields to origins of life research, stating that they have allowed researchers to examine systems like this in unprecedented detail.
“This outlines a very systematic approach to delineating how membraneless compartments, like polyester-based microdroplets, interact with various cations, many of which are central to prebiotic processes, including RNA oligomerization, replication, catalysis, and compartment formation and function,” added Rajamani.
“This is very pertinent given the prevalence of salts (some in high concentrations) in early Earth niches that are thought to have led to life’s emergence,” she continued. “Importantly, they have used a very sensitive technique (ICP-MS) to discern the salt concentration directly in the droplets; a novel and informative element of this study with ramifications for prebiotic chemistry and related research.”
A step forward
The team’s findings were intriguing. As Chen explained, “We found that polyester microdroplets could uptake salts, and that different salts are taken up at different rates.” Moreover, the salts tended to accumulate near the charged surfaces of the droplets, resulting in an overall neutralization of the droplet’s surface charge.
“Because of this, the droplets then stopped repelling one another, and instead started to coalesce,” added Chen. “This is significant as it could explain one way in which primitive polyester microdroplets could have grown — a hallmark of life.”
These findings shed light on the fact that even slight variations in salt uptake can significantly influence the structure of these possible protocells. This observation also offers a potential explanation for the diverse chemistries observed in primitive systems that emerged in different aqueous environments, ranging from freshwater to oceanic to hypersaline under-ocean brines.
“To zero in on membraneless coacervate systems that can tolerate and potentially function in the presence of high salt concentrations is completely non-trivial,” added Rajamani. “This will help zero in on analogue environments where prebiotically important reactions would have been readily feasible. Such studies not only help find answers to pressing questions in the field of [the origins of life], but also have direct implications for habitability related research on other promising solar system bodies and exoplanets.”
“We still know very little about the conditions that led to life, and the variable space is so wide,” added Fraccia. “Thus, any efforts dedicated to test physical–chemical conditions […] can help us in drawing ‘boundary conditions’, that can […] drive the search for life in other parts of the Universe.”
While these results are a promising step forward, some unanswered questions remain. For example, what is the significance of the emergence of different salt uptake rates? Could this have been related to the emergence of biological systems to maintain different salt ratios, such as the potassium and sodium ion pumps?
But Jia and Chen say they are not done yet. “Without going into too much detail, we hope to continue studying the stability and structure of the polyester microdroplets, while also probing other emergent functions as well as polyester chemical evolution,” said Jia.
“We hope that this could be the starting point for future exploration on other primitive environments affecting the stability and functionality of polyester microdroplets,” added Chen. “[This understanding] could also provide valuable information about potential habitability beyond our solar system.”
Reference: Chen Chen, Tony Z. Jia, et al., Spectroscopic and Biophysical Methods to Determine Differential Salt-Uptake by Primitive Membraneless Polyester Microdroplets, Small Methods (2023). DOI: 10.1002/smtd.202300119
Feature image credit: Chen Chen, Tony Jia, et al.