 Researchers from China, Germany, and Portugal discovered that a trilayered nanomembrane greatly enhances the properties of Li–O2 batteries. With a power conversion efficiency (PCE) of 86%, a stable cycling performance for 269 cycles, and a charge overpotential as low as 0.2 V, the batteries show promising properties for the application in low-emission electric vehicles.
Researchers from China, Germany, and Portugal discovered that a trilayered nanomembrane greatly enhances the properties of Li–O2 batteries. With a power conversion efficiency (PCE) of 86%, a stable cycling performance for 269 cycles, and a charge overpotential as low as 0.2 V, the batteries show promising properties for the application in low-emission electric vehicles.
Li–O2 batteries have a high energy density, which makes them suitable to improve the driving range of low-emission electric vehicles. However, in conventional devices, the cycling stability and PCE of these batteries is low. The reason for this poor performance lies in a high overpotential due to insoluble Li2O2, which is formed during discharging and interferes with the battery architecture.
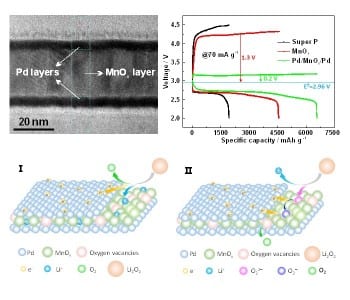
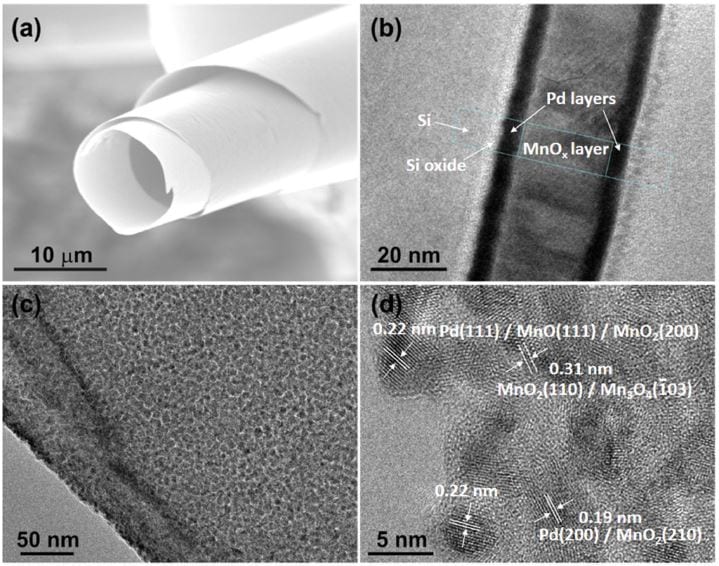
In their recent approach, Xueyi Lu et al. introduce a Pd/MnOx/Pd nanomembrane onto the cathode. This shields the cathode from Li2O2 and catalyzes its removal during charging. The membrane components Pd and MnOx synergistically catalyze the processes that occur during recharging of the battery. The oxygen vacancies in the membrane facilitate binding of intermediate structures during the decomposition of Li2O2 and the Pd components accelerate electron transport. The nanomembrane is fabricated by rolled-up technology, which results in a higher tolerance to stress cracking during charge–discharge cycles. Further, the decrease of active sites due to aggregation is avoided, since 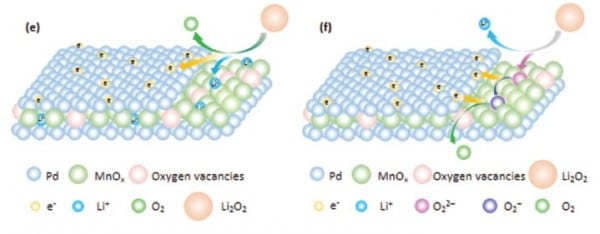 the nanomembranes consist of grains instead of nanoparticles.
the nanomembranes consist of grains instead of nanoparticles.