Microfluidic generation of three-dimensional hydrogel gradients to modulate hematopoietic stem cell culture microenvironments
Hematopoiesis is the process where all the body’s blood and immune cells are generated from a small number of hematopoietic stem cells. These events take place in unique parts of the bone marrow termed ‘niches.’ The genetic information required to direct hematopoietic stem cell behaviors is contained within its DNA, but external signals from the niche are required to trigger these events. And while hematopoietic stem cells are responsible for producing nearly one trillion hematopoietic cells per day such as platelets and immune cells, mutations in this process are responsible for hematopoietic pathologies such as leukemia. However, it is difficult to study these events inside the bone marrow. Understanding how niche signals shape hematopoiesis and control the onset and expansion of pathologies requires new tools to allow researchers to examine these complex processes outside of the body.
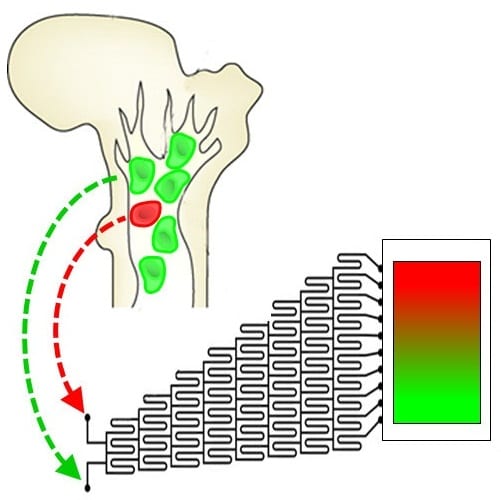
A long term project to build an artificial bone marrow that provides the correct sequence of niche signals to grow hematopoietic stem cells in the laboratory is the focus of research led by Brendan Harley at the University of Illinois. In recent work, they describe a microfluidic mixing platform to create small volume, three-dimensional hydrogels containing overlapping patterns of cell and matrix constituents inspired by the native hematopoietic stem cell niche. This proof-of-principle study demonstrates an approach that can be used to generate hydrogels containing opposing gradients of fluorescent microspheres, osteoblasts (a primary bone marrow niche cell), primary murine hematopoietic stem and progenitor cells, and combinations thereof in a manner independent of hydrogel density and cell/particle size. Additionally, the work demonstrates multiple forms of analysis, at both single cell and populational levels can be used to locally assess cell response to the gradient environment. In summary, the approach allows creation of stable gradients of cell and material cues within a single, optically-translucent biomaterial in order to mimic known heterogeneities across the bone marrow and enable a range of investigations regarding how microenvironmental signals impact hematopoietic cell fate.
Check the most downloaded papers from Advanced Healthcare Materials, the journal that recently published this research.