 For decades, the gold-standard assay system for detecting and quantifying target molecules has been ELISA – enzyme-linked immunosorbent assay. Though many different formats of ELISA exist, they generally rely on two biological components: antibodies that bind to the target, and enzymes capable of triggering a visible color change upon addition of a substrate.
For decades, the gold-standard assay system for detecting and quantifying target molecules has been ELISA – enzyme-linked immunosorbent assay. Though many different formats of ELISA exist, they generally rely on two biological components: antibodies that bind to the target, and enzymes capable of triggering a visible color change upon addition of a substrate.
Despite its popularity, ELISA suffers from various shortcomings. Manufacturing, purifying and characterizing a new antibody takes a minimum of 2–3 months. This process often involves harvesting blood from animals, raising ethical concerns. Performing the assay requires trained personnel, as ELISA involves a series of steps, including blocking, washing and addition of enzyme substrate. Perhaps most debilitating is the inherently low stability of antibodies and enzymes used within ELISA. As biological molecules, these have limited shelf-life and require reliable cold storage, which is not always available in developing countries.
In ChemNanoMat, Stanislav Piletsky and his colleagues developed a completely abiotic alternative to ELISA, replacing both antibodies and enzymes with fluorescent molecularly imprinted polymer nanoparticles (nanoMIPs). Due to the synthetic nature of nanoMIPs, they are cheap and easy to synthesize, stable at a broad range of conditions, and have long shelf life without refrigeration. Furthermore, the assay format that they developed requires only one simple step (adding the test sample), and can therefore be performed with minimal training.
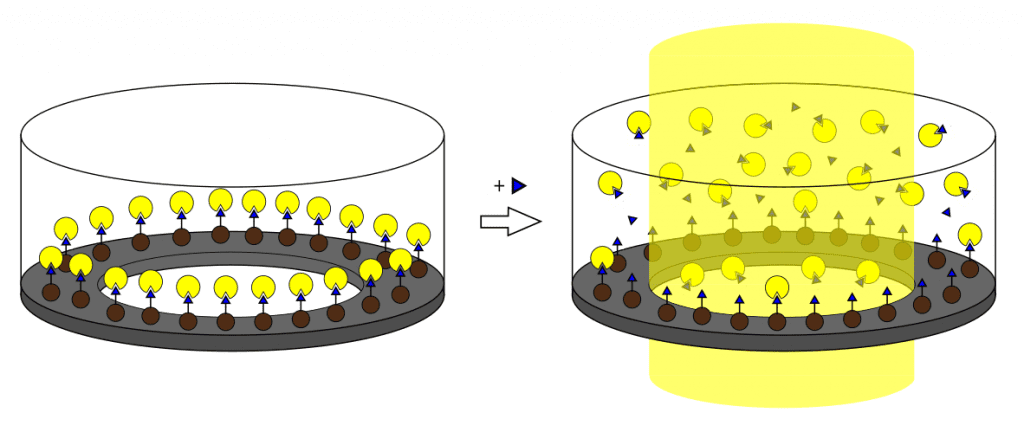
The assay format relies on displacement and detection of fluorescent nanoMIPs, triggered by addition of free target molecules. Instead of traditional physical adsorption, the team used magnetic microtiter plate inserts to conveniently immobilize target-conjugated magnetic nanoparticles. Fluorescent nanoMIPs bound to the immobilized target are shielded from the plate reader, resulting in an absence of fluorescence. Addition of free target displaces the nanoMIPs, causing an increase in fluorescence proportional to the target concentration.
Text kindly provided by the authors of the original manuscript.