Chlorinated organic pollutants such as trichloroethylene (TCE) and polychlorinated biphenyls (PCBs) in water streams are a big problem as they are often neurotoxic or mutagenic for wildlife and humans. Metallic nanoparticles have been used in recent times to degrade these pollutants by catalyzing their hydrogenation to harmless compounds, but they often tend to aggregate due to their magnetic properties, which in turn reduces their reactivity.
Immobilizing the nanoparticles prevents aggregation, but again often at the expense of reactivity. Hydrogels may offer a helping hand here, since they are tunable environments whose properties can be tailored first to create the nanoparticles in situ, and then optimized for the catalytic reaction.
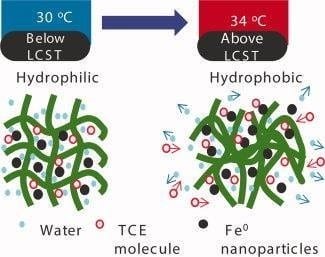
This is exactly what Xiao and coworkers at the University of Kentucky have done, in a recent paper published in the Journal of Applied Polymer Science, in which they have used N-isopropylacrylamide and its copolymers with acrylic acid, P(NIPAAm-AA), to entrap and synthesize reactive iron and iron/palladium nanoparticles. When temperature is varied from 30 to 34 degrees Celsius, the hydrogels change their character from hydrophilic to hydrophobic, as can be seen in the figure, and this in turn generates threefold increase in reactivity of the entrapped nanoparticles, compared to twofold increase for the free nanoparticles in the same temperature range. This happens because, since the hydrogel is hydrophobic at the higher temperature, it interacts strongly with hydrophobic compounds such as TCE, and it shrinks expelling some of the water, with the end result of enhancing the overall reaction rate.