 Nature has developed the unique capability to produce and regulate proteins via two main processes: biosynthesis based on genetic control or highly specific chemical modification after biosynthesis (post-translational process). These processes have inspired scientists and have advanced genetic engineering and protein chemistry techniques so that entirely novel proteins, such as fusion proteins and chemically modified proteins, can be prepared.
Nature has developed the unique capability to produce and regulate proteins via two main processes: biosynthesis based on genetic control or highly specific chemical modification after biosynthesis (post-translational process). These processes have inspired scientists and have advanced genetic engineering and protein chemistry techniques so that entirely novel proteins, such as fusion proteins and chemically modified proteins, can be prepared.
Fusion proteins consist of different proteins or protein domains. Highly potent and specific protein therapeutics such as novel antibody-enzyme hybrids have been prepared as fusion proteins for the treatment of major diseases such as cancer.
Enhanced potency of clinically-used anti-cancer drugs has been achieved when applied in combination with different proteins, which is very attractive for tumors resistant to existing treatments. However, in order to further enhance the potency of fusion proteins or to impart completely novel features, entirely new approaches are urgently needed. One could envision combining genetic engineering, specific biorecognition and site-directed chemical modifications to achieve sophisticated protein hybrids with tailored features that could not be achieved solely using genetic engineering techniques.
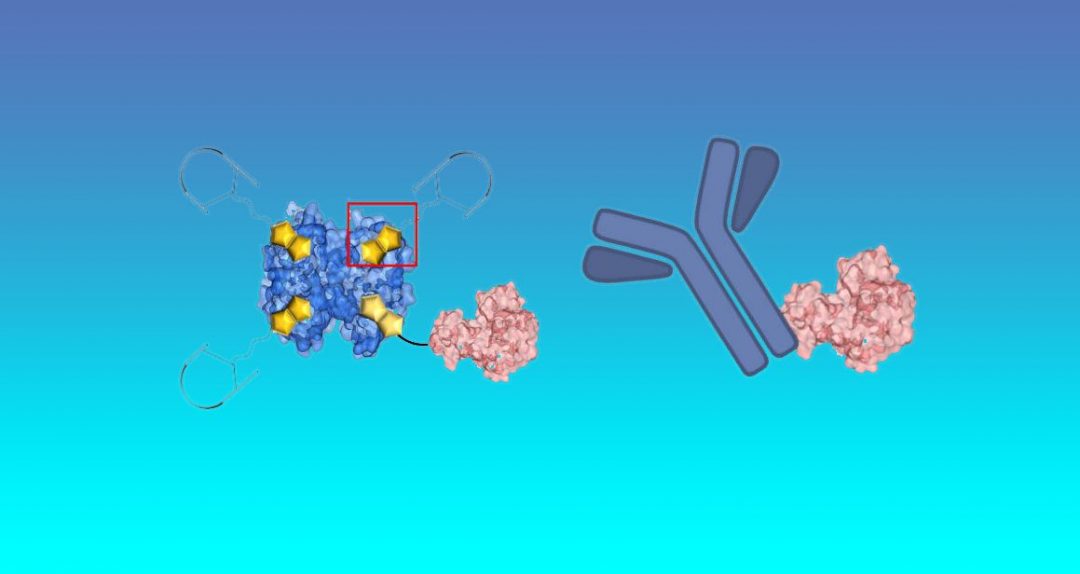
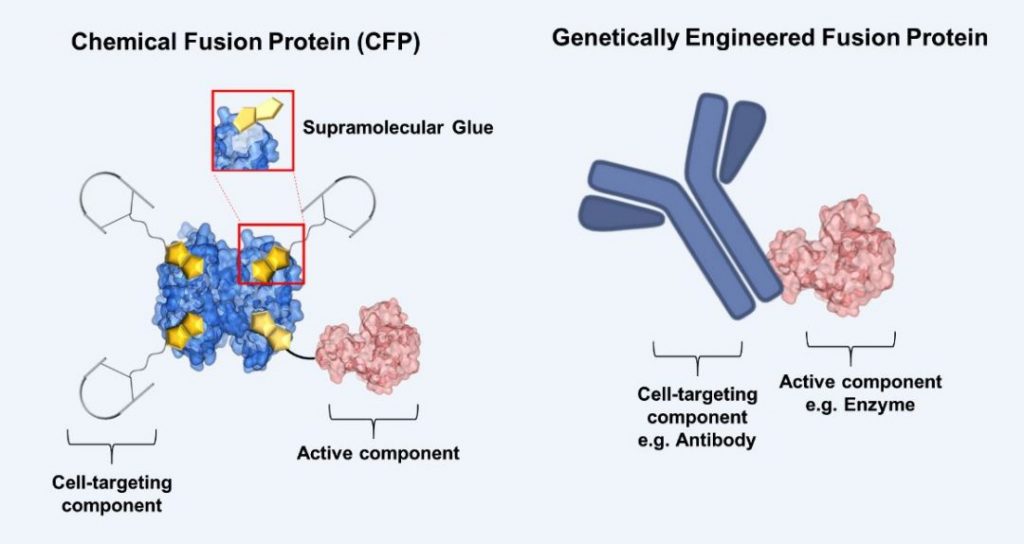
Seah Ling Kuan, Tanja Weil, Thomas Simmet and Holger Barth from the Max Planck Institute of Polymer Research and Ulm University worked together to devise a platform that integrates protein chemistry and recombinant molecular biology approaches. The highly multidisciplinary team uses a supramolecular glue based on vitamin H (biotin) and its natural binding protein, commonly known as the avidin-biotin technology, to formulate a completely new chemical fusion protein (CFP), which cannot be accomplished by biotechnological means alone. The supramolecular glue binds together (1) about three copies of a peptide that targets specific membrane receptors on cancer cells and (2) a Rho-protein inhibiting toxin, which is known to inhibit blood vessels growth and, at the same time, allows cancer cells to be more sensitive to anti-cancer drugs when used in combination.
Notably, they investigated the potency of the CFP in chick embryo models and compared it to an approved antibody treatment (bevacizumab), as well as investigated if it enhances the drug efficiency of the clinically used anticancer drug, doxorubicin. This method merges the best of chemical and biochemical tools and lays a foundation for creating highly effective protein therapeutics for cancer therapy.