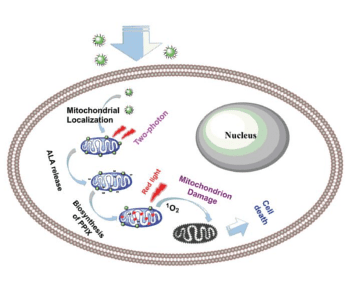
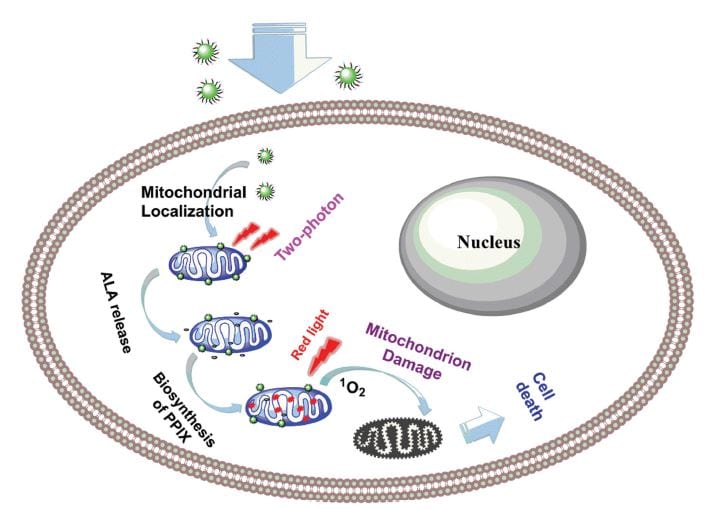
 A carbon-dot nanosystem is developed, which releases a photosensitizer bioprecursor in cancer cells upon near-infrared irradiation. The nanodots are taken up by cancer cells and accumulate in mitochondria, where the bioprecursor is metabolized to form the photosensitizer protoporphyrin IX (PPIX). This photosensitizer can induce cell death when it is irradiated by near-infrared light. The nanosystem avoids cellular uptake of bare photosensitizer molecules. Therefore, limitations due to the bioavailability and biocompatibility of such molecules are overcome and side effects due to their toxicity are reduced.
A carbon-dot nanosystem is developed, which releases a photosensitizer bioprecursor in cancer cells upon near-infrared irradiation. The nanodots are taken up by cancer cells and accumulate in mitochondria, where the bioprecursor is metabolized to form the photosensitizer protoporphyrin IX (PPIX). This photosensitizer can induce cell death when it is irradiated by near-infrared light. The nanosystem avoids cellular uptake of bare photosensitizer molecules. Therefore, limitations due to the bioavailability and biocompatibility of such molecules are overcome and side effects due to their toxicity are reduced.
Photodynamic therapy (PDT) is a minimally invasive treatment for several cancer types. Cell death is induced via generation of reactive oxygen species, such as singlet oxygen or superoxide anion radicals by irradiating a photosensitizer molecule. With their nanosystem, Shuizhu Wu, Jianrong Qiu, and co-workers generate photosensitizer in mitochondria by releasing 5-aminolevulinic acid (5-ALA), which is metabolized to PPIX.
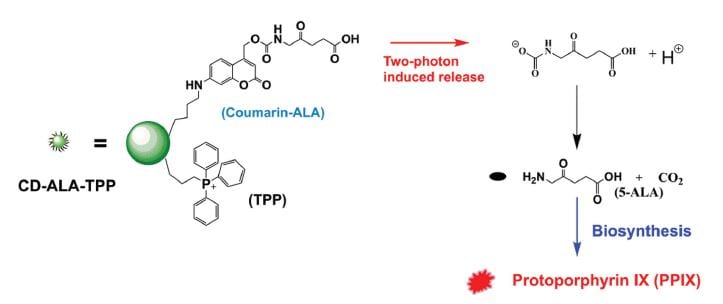 The developed nanosystem consists of a carbon nanodot, which serves as carrier for the mitochondria-targeting cation triphenylphosphonium (TPP) and the bioprecursor 5-ALA linked to coumarin. Coumarin can release 5-ALA upon two-photon irradiation at 800 nm. TPP is efficiently taken up by cells and accumulated in close proximity to mitochondria, which leads to the formation of PPIX in these cell compartments. 5-ALA’s low cell penetration ability and low stability in biological fluids are overcome by this approach. Additionally, the release of 5-ALA is spatially controlled by irradiation and, thus, can be induced selectively. The authors propose that their system can be exploited to develop photodynamic-therapy-related applications.
The developed nanosystem consists of a carbon nanodot, which serves as carrier for the mitochondria-targeting cation triphenylphosphonium (TPP) and the bioprecursor 5-ALA linked to coumarin. Coumarin can release 5-ALA upon two-photon irradiation at 800 nm. TPP is efficiently taken up by cells and accumulated in close proximity to mitochondria, which leads to the formation of PPIX in these cell compartments. 5-ALA’s low cell penetration ability and low stability in biological fluids are overcome by this approach. Additionally, the release of 5-ALA is spatially controlled by irradiation and, thus, can be induced selectively. The authors propose that their system can be exploited to develop photodynamic-therapy-related applications.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.