The process of metastasis, in which cancer cells migrate to distant organs from the primary tumor region, is the key pathological event responsible for 90% of all of the cancer-related deaths.
The critical early steps of the metastatic cascade of events, namely invasion and intravasation, involve many complex biological processes. These processes require better understanding in order to develop advanced targeted therapies. Unfortunately, the conventional methods to study these processes, such as animal models for example, often fail at isolating the specific studied process element due to a shear abundance of parallel-occurring events and participating factors.
Microfluidic technologies offer methods to dissect these processes by mimicking the cancer cell microenvironment. These technologies enable organizing cells in 3D to create miniature tumors on a chip that can truly recapitulate the tumor microenvironment and address the limitations of current animal models and conventional assays.
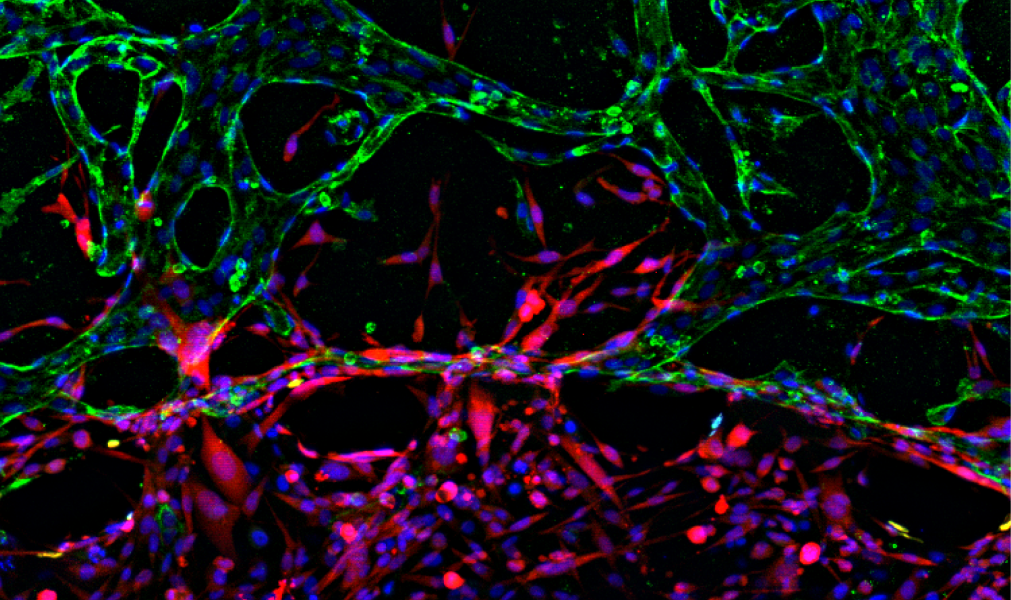
In their article in Advanced Healthcare Materials scientists from the US developed a novel microfluidic platform comprised of concentric three-layer regions containing breast cancer cells, stroma, and vasculature to mimic the tumor microenvironment. This device is capable of simultaneous observation of vascular network formation and breast cancer cells’ invasion and intravasation.
The authors demonstrate that presence of the spontaneously formed vasculature drastically enhanced metastatic cancer invasion from the primary tumor region into the adjacent stroma layer. Notably, cancer cells could be visualized entering into the vasculature allowing for future in-depth studies of tumor and vascular interactions.
Furthermore, they showed that cancer cells had a significantly reduced diameter and also, an increased permeability of the vessels, demonstrating consistent pathology with previous animal studies. Most importantly, the scientists have identified major signaling molecules within their microfluidic platform that could govern cancer invasion in the presence of the vascular networks.
Future investigation of these signaling cues involved in metastasis by using the microfluidic device will give unique insights into critical biological events governing these treacherous processes while paving the way for the discovery of efficient cancer therapeutics.