We are all familiar with the beauty of diamonds and their role as beautiful ornamental stones that are said to “last forever.” But outside of jewelry, diamond possess a range of extraordinary properties that make it an exceptional material.
Among them is its mechanical stability and its reputation as one of the hardest known materials in the world. As such, diamonds are used extensively in industrial applications as they are quite effective for polishing, cutting, and drilling.
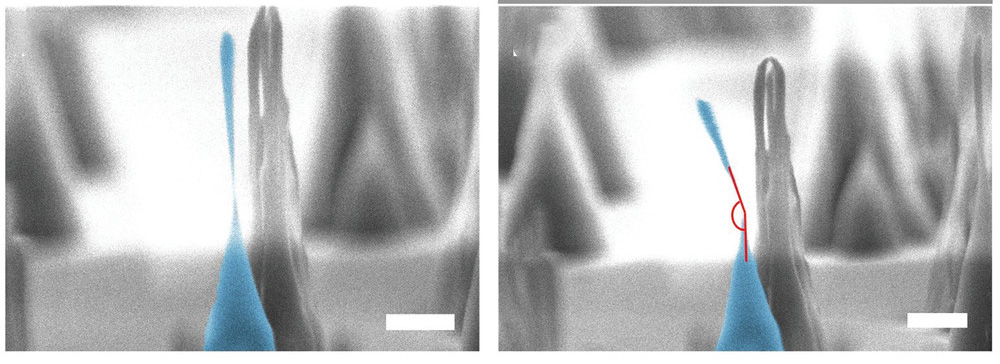
But now a team of Australian scientists have turned this idea of diamond’s indisputable hardness on its head; having discovered that diamond can actually be bent and deformed … that is, on the nanoscale.
According to the study — which was recently published in Advanced Materials — the “mechanical properties of bulk materials can be altered dramatically when the material dimensions are reduced to the micro‐ and the nanoscale”, opening the door for a range of new possible applications in areas such as sensor development, defense, and energy storage.
“Diamond is the frontrunner for emerging applications in nanophotonics, microelectrical mechanical systems and radiation shielding,” said Blake Regan, Ph.D. student at the University of Technology Sydney (UTS) and the study’s lead author. “This means a diverse range of applications in medical imaging, temperature sensing and quantum information processing and communication.
“It also means we need to know how these materials behave at the nanoscale — how they bend, deform, change state, crack. And we haven’t had this information for single-crystal diamond.”
The team demonstrated that nanoscale diamond pillars could undergo not only elastic deformation and fracture, but also a new form of plastic deformation that depends on the nanopillar dimensions and crystallographic orientation of the diamond.
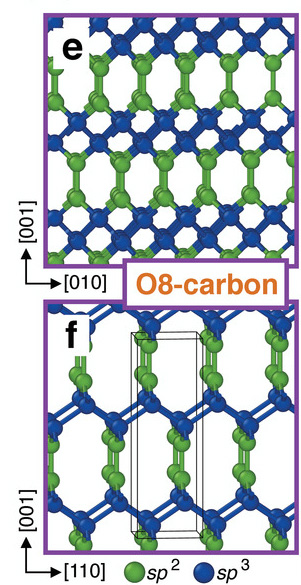
The plastic deformation can be explained by the emergence of a new phase of ordered carbon called O8‐carbon that is localized in the deformed regions of the bent nanopillars. In diamond, each carbon atom forms four bonds to four other carbon atoms due to “orbital hybridization”, which is the concept of mixing atomic orbitals into new hybrid orbits (with specific energies and geometries) for forming chemical bonds. In diamond, carbon atoms adopt what is termed an sp3 hybridization.
In this new phase of carbon, a 50:50 mixture of two types of atom hybridizations occurs between sp2 (graphite‐like) and sp3 (diamond‐like) hybridizations.
“The results demonstrate unprecedented mechanical behavior of diamond, and provide important insights into deformation dynamics of nanostructured materials,” stated the authors in their study.
According to UTS Professor Igor Aharonovich: “These are very important insights into the dynamics of how nanostructured materials distort and bend and how altering the parameters of a nanostructure can alter any of its physical properties from mechanical to magnetic to optical. Unlike many other hypothetical phases of carbon, 08-carbon appears spontaneously under strain with the diamond-like bonds progressively breaking in a zipper-like manner, transforming a large region from diamond into 08-carbon.
“The potential applications of nanotechnology are quite diverse. Our findings will support the design and engineering of new devices in applications such as super-capacitors or optical filters or even air filtration.”
Research article found at: B. Regan, et al. Advanced Materials, 2020, doi.org/10.1002/adma.201906458