Our body has the impressive ability to heal itself after a minor injury, such as a cut to the skin, a pulled muscle, and even broken bones. But unlike the gecko, which can completely regrow a lost tail, not all human cell types can regenerate themselves.
Neurons that control our muscle movements known as motor neurons or motoneurons cannot “rebuild” after suffering damage. Two therapeutic approaches to repairing this type of nerve damage have been widely studied in the clinic: implantable electronic devices that simulate the electrical signals of motoneurons, and transplanting stem cells to the tissue to hopefully reconnect it to a healthy part of the damaged nerve.
Both approaches show promise but have their drawbacks, and have not yet achieved a full muscle-nerve reconnection that lasts. “Due to the fundamentally different nature of biological tissue compared to that of materials used in implants, this interface is often far from perfect, and subject to inflammation, scarring, and implant damage,” said Damiano Barone, clinical lecturer in Neurosurgery at the University of Cambridge.
“[On the other hand,] while the integration of transplanted cells is good, there is little way to control the transplanted population once it is in the body [and,] in the nervous system, cells on their own are unable to restore complex networks and therefore restore function,” he continued.
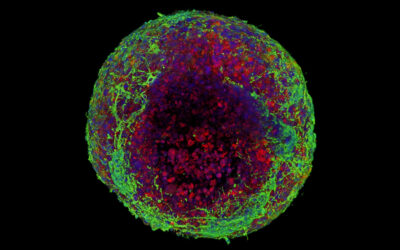
In collaboration with scientists from BitBio, Barone and his team built a biohybrid device that contains both electronic and biological components to improve implant integration and better guide connection with a damaged nerve. In a recent study, they built this implantable device, which was covered in a layer of cells to create a biological interface to better connect the device to the tissue.
“Our biohybrid technology combines the best of both of these approaches [referring to implantable devices and cell transplantation],” said Barone. “By including a cell layer in the implant, the tissue can fully grow and integrate with the implant to produce a very stable interface. This allows us to fully realize the potential of neural interfaces – recording simultaneously from many nearby regions of tissue in a very stable way.”
Finding the right materials
When developing an implantable device it is critical that it be biocompatible to avoid rejection. This commonly occurs as a result of activation of the immune system, which considers it a “foreign body”. The device also needs to be small to avoid interfering with surrounding organs, and flexible so it can adapt to the tissue shape where it is implanted.
Despite their small size, to function implants need to incorporate the necessary electronic equipment — in this case, tiny electrodes called microelectrodes that act as contact points to record electrical signals from tissue.
The researchers say they chose to work with a parylene-based microelectrode array that meets all these needs while allowing a fine, precise recording of electrical signals coming from connected nerves and a high-resolution electrical connection to the muscle.
“Parylene refers to the material which makes up the body of the implant, as well as the insulator around the electrodes,” said Alejandro Carnicer-Lombarte, a postdoctoral researcher in the Department of Engineering at the University of Cambridge, and one of the co-first authors in the study. “We use this because it is very biocompatible and can be fabricated in very thin layers, resulting in an extremely flexible device. This allows the implant to bend around tissue without breaking or damaging the body.”
A layer of cells
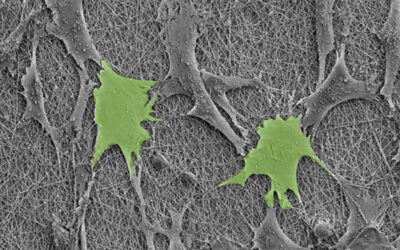
As the biological component the team chose to work with a type of stem cell called induced pluripotent stem cells (or iPSC for short), which had been transformed into muscle cells called skeletal myocytes.
“The choice of iPSC was driven by the specific application for which the biohybrid interface was designed,” explained Carnicer-Lombarte. “We specifically designed the device to interface into a nerve-controlling movement of the rat paw. As this is driven by spinal motoneurons normally innervating muscles, a myocyte iPSC layer was the ideal biohybrid device population.”
The team combined these iPSCs with the microelectrode array together in a Petri dish and induced their transformation from stem cells to grown muscle cells. After eight days, the array was covered with the cells and ready to implant.
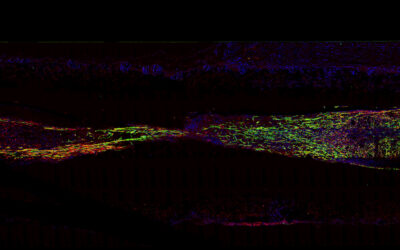
After four weeks, the researchers found biological connections between the device and the tissue in the forearm of rats, suggesting the successful integration of the device with the damaged nerve. The scientist also tested the biohybrid implant’s ability to receive electrical signals with connected nerves by stimulating a nerve located a few centimeters upstream of the implant and found that it was able to receive the electrical signal, suggesting a healthy nerve physiology above the point of damage.
Last, they wanted to test the biohybrid device in walking rats. By the fourth week post-implantation, the team found a correlation between paw movement and the device’s electrical activity, suggesting it was receiving the information destined for the muscles in the paw from the healthy section of the damaged nerve.
Bringing biohybrid implants to the clinic
Although the device has not been tested in humans yet, the team is optimistic that the properties of each of its components support its safety and functionality.
“Current research indicates that versions of human iPSCs edited to prevent immune rejection and facilitate transplantation may be available in the future, and would make an ideal cell component in biohybrid technology,” said George Malliaras, professor of Technology at University of Cambridge and co-lead author of the study. “[Moreover] both implantable neural interfaces and (stem cell-derived) cell transplants are well explored at the clinical level. This makes us confident on the feasibility of moving biohybrid technology into clinical testing in the not-too-far future.”
The authors explained that they are also working on biohybrid devices for other applications, one of which involves replacing muscle cells with neurons and developing a device that could work as an interface to restore function in a damaged brain.
As biohybrid implantable devices continue to demonstrate their potential, their future in clinical applications looks promising, offering new hope for patients in need of innovative medical solutions.
Reference: Amy E. Rochford, Alejandro Carnicer-Lombarte, et. al., Functional neurological restoration of amputated peripheral nerve using biohybrid regenerative bioelectronics, Science Advances (2023). DOI: 10.1126/sciadv.add8162