Advanced combination therapy, e.g. traditional chemotherapy in combination with novel gene therapy, is an emerging treatment strategy which involves administering two or more different therapeutic agents to maximize beneficial effects. This approach is particularly applicable for aggressive diseases such as cancer, for which current therapeutic options are very limited.
Such a potentially powerful therapeutic modality, however, is greatly hampered by the lack of desirable drug delivery systems that can accommodate various agent payloads, prevent degradation, avoid serum susceptibility, and improve bioavailability. By applying boronic acid coupling and host-guest chemistry, Jun Feng and collaborators have now addressed this challenge with a versatile pH-responsive drug/gene co-delivery nanoplatform.
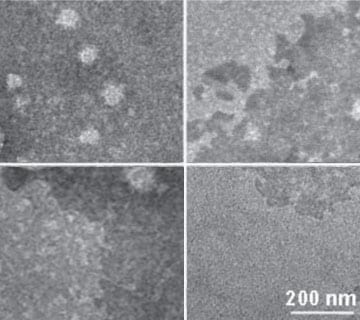
The system is constructed on the stable self-assembly between hydroxyl-rich γ-cyclodextrin (γ-CD) and phenylboronic acid-modified oligoethylenimine (OEI-PB) at neutral conditions. The fascinating merit of this construction originates from the unique property of γ-CD to accommodate hydrophobic agents via host-guest chemistry, as well as the acid-labile feature of boronic acid-diol linkage by which the drug/γ-CD inclusion would readily undergo acidity-induced detachment from the DNA/vehicle polyplexes. As a result, a spectrum of antitumor drugs can be easily incorporated into the cationic OEI-PB-γ-CD vehicles along with anionic DNA.
The intelligent drug/gene co-delivery nanoplatform not only can facilitate cellular entry of drug payloads into tumor cells via an endocytosis pathway, but also is responsive to acidic lysomal/endosomal compartments and/or tumor microenvironments with a rapid drug release so as to effectively elicit therapeutic actions. Another important purpose of the design is to enable the formed vehicle/DNA nanoformulation surrounded densely by γ-CD moieties in order to bio-mimic the carbohydrate-rich cell surface. Due to such a so-called “surfacial saccharide camouflage”, the DNA/vehicle polyplexes exhibited strong serum resistance capability to overcome serum-susceptible drawbacks (e.g. reduced cellular uptake, aggravated protein adsorption and inhibited transfection) frequently associated with synthetic gene vehicles.
Consequently, OEI-PB-γ-CD vehicles afforded significant 25-fold higher gene transfection efficiency over the golden standard of PEI25K in the presence of 30% serum. More importantly, drug inclusion did not alter the excellent serum-compatible transfection efficiency mediated by OEI-PB-γ-CD vehicles. This study by combining the advantages of phenylboronic acid coupling and host-guest chemistry may provide a novel and versatile strategy to develop safe and effective nanoplatforms for combinational therapies against tumor diseases.