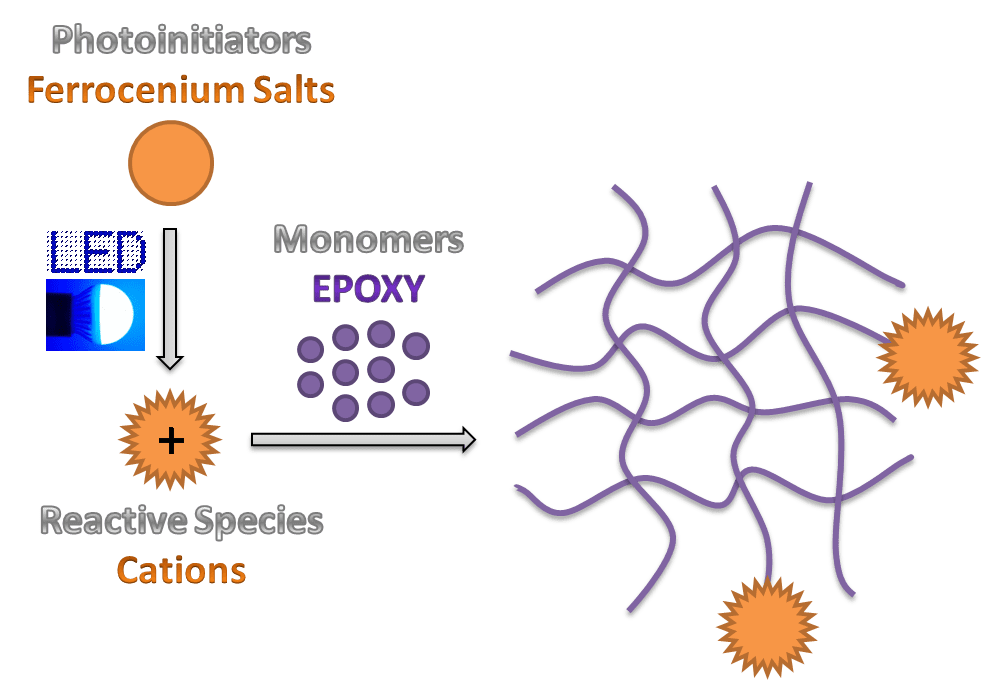
Professor Jacques Lalevée and his research team from the Institut de Science des Matériaux de Mulhouse (CNRS, France) have been developing new cationic photoinitiating systems and photoinitiators, which can be used for polymerization. It has been found that ferrocenium salts exhibit broad visible light absorption and can behave as one-component photoinitiators for cationic photopolymerization operating under near UV and visible LEDs.
There are many economic, environmental and production benefits related to using light as a trigger for processes. The ability to generate polymers, in a non-invasive manner, without the need for extra chemical input, makes photopolymerization a very attractive candidate for the preparation of various industrially viable materials (coatings, dental composites, 3D printing products, etc.).
In this technique, initiating species are generated by a photochemical reaction upon irradiation at room temperature and subsequently a reaction with monomer units takes place. Photopolymerization is thus well-suited for producing 3D polymer networks upon crosslinking by rapidly transforming specially-formulated reactive liquids into various functional solids.
In comparison to widely investigated free radical polymerization, cationic polymerization is undoubtedly interesting due to its advantages such as oxygen insensitivity, low shrinkage, post curing effect and possible use of renewable monomers. However, most of cationic photoinitiating systems require harmful high-energy and short-wavelength UV light to operate and induce the polymerization. Hence it is important to develop new systems which could react under milder photoreaction conditions.
Recently, light-emitting diodes (LEDs) have been emerging as potential irradiation sources to replace traditional mercury UV lamps. Some of their advantages include higher light output, greater environmental compatibility, improved operating efficiency, and lower cost and energy consumption. Therefore, it would be desirable to design and develop high-performance cationic photoinitiators.
In the article, which has been recently published in Macromolecular Chemistry and Physics, the authors give an overview of the field and highlight the most promising initiating systems for cationic photopolymerization.