Prostate cancer (PCa) remains one of the leading causes of cancer-related deaths in men. Prostate biopsy, generally used to diagnose prostate cancer, is invasive and usually associated with patient discomfort and potential complications such as infections. Despite the tremendous progress in research over the years, a suitable minimally invasive PCa biomarker is yet to be discovered. Although serum prostate specific antigen (PSA) represents the most used prostate biomarker to date, a major drawback is related to its lack of specificity; altered levels of this protein being also observed in benign prostatic hyperplasia and inflammation, as well as after physical manipulation of the prostate or lifestyle factors including carbohydrate intake or body weight. Considering that PSA still remains a useful marker, despite leading to overdiagnosis and overtreatment of prostate cancer, increasing its reliability by including additional markers is highly desirable, albeit challenging.
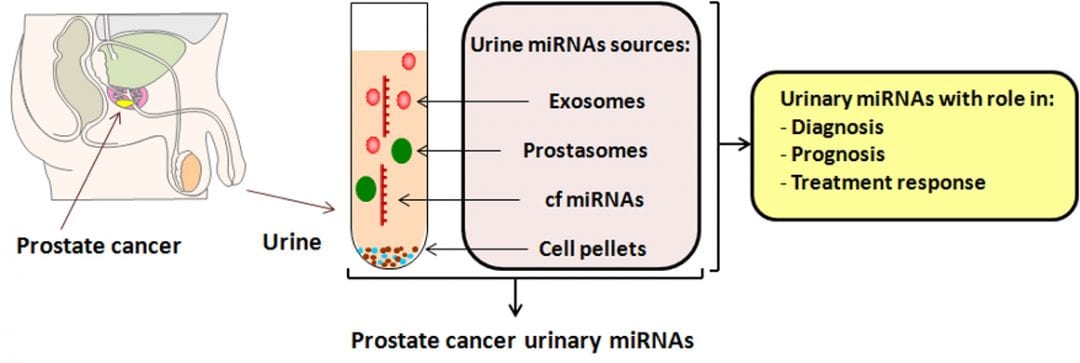
The discovery of microRNAs (miRNAs) has drawn attention towards this class of small noncoding RNAs that regulate the activity of protein coding genes and play essential roles in a broad range of biological processes including cancer. Due to their high stability in different types of tissues and body fluids and deregulated expression in various cancers, miRNAs have shown great potential to identify new biomarkers for cancer detection and prognosis. In PCa, the majority of miRNA-based biomarkers were evaluated in blood, other relevant body fluids such as urine being less investigated, although urine is easily collected through totally non-invasive techniques.
In a recent review article in WIREs RNA, Balacescu et al. summarized the role of urinary miRNAs as a new class of stable and prostate-specific biomarkers, and highlighted how these urinary biomarkers could improve the specificity of PSA for prostate cancer diagnosis, prognostic and treatment response. A high degree of inconsistency among reports has been observed, which could be due to several analytical aspects, starting with different urinary fractions used for analysis and continuing with the employment of various analytical platforms and methods of statistical analysis. However, a few microRNAs were found to be consistently dysregulated in the urine of PCa patients, which alone or together with serum prostate-specific antigen seem to improve the diagnostic power. Because of the poor standardization of methods used to assess these urinary biomarkers, the authors also presented the technical challenges that have to be considered when evaluating the urinary miRNAs, concluding that the results obtained so far warrant further confirmation by larger prospective studies, preferably using a standardized protocol for analysis. So to answer the question in the title, it seems that we are not there yet, but we are on the right track.
Kindly contributed by the Authors.