Conventional thermosetting photopolymers used in current industrial settings are typically composed of multifunctional (meth)acrylates and/or epoxy systems. These photopolymers are commonly prepared by photo-initiated radical chain-growth or epoxy-based cationic polymerizations, which exhibit rapid reaction kinetics and yield glassy materials with high mechanical stiffness and strength. A major undesired characteristic of such highly cross-linked photopolymer networks is however their inherent brittleness that negatively affects polymer performance and limits their applications. Hence, designing thermosetting photopolymers that feature high stiffness and strength, as well as thermoplastic-like ductility is a now highly prioritized research direction in the field.
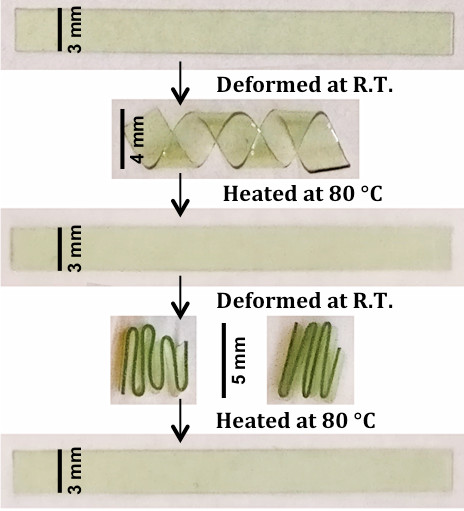
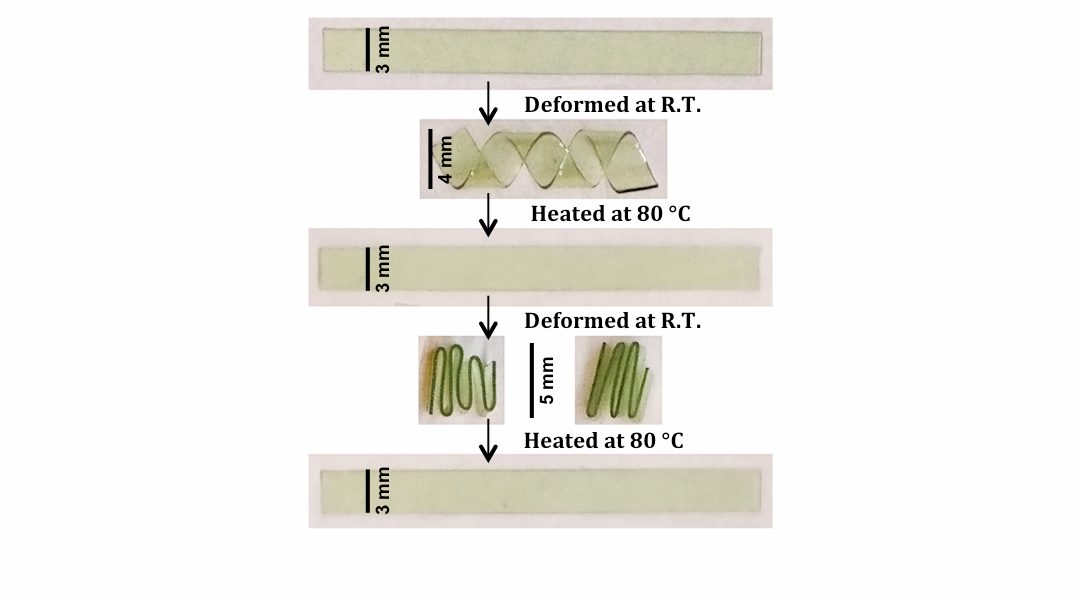
Multiple shape memory-recovery behavior of the glassy-polymer. The specimen was deformed at glassy state followed by subsequent thermal recovery cycle.
A common strategy is to incorporate various toughening agents (e.g., carbon nanotubes, platelets, low molecular weight linear elastomers, block polymers, and core/shell particles); though this leads to a positive impact on the photopolymer toughness, an increase in viscosity, light scattering, or opacity is a most typically observed side effect. Other approaches are the use of (i) addition fragmentation chain transfer agents, (ii) thiol-ene/-yne based step-growth polymers, (iii) urethane-based glassy co-polymers, or the formation of glassy cross-linked photo-polymer networks by ring opening metathesis polymerization (ROMP). However, due to a limited control over reaction kinetics, a more facile and efficient approach to thermosetting polymers is still highly desired.
In their recent work, Prof. Bowman and co-workers designed glassy cross-linked thermosetting photopolymers with unique ductile behavior based on triazole-containing networks formed by two independent photo-“click” chemistry approaches, namely, either by photo-initiated copper(I)-catalyzed cycloaddition of monomers containing azide and alkyne groups (CuAAC photo-polymerization), or by photo-initiated thiol-ene polymerization of monomers containing triazole moieties.
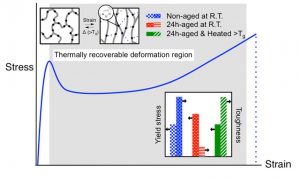
Schematic illustration of the triazole‐based networks before and after deformation and the ductile behavior of the networks. More information here.
In spite of their highly cross-linked nature, these triazole-containing thermosetting photopolymers exhibit a combination of remarkable features, that is, dramatically enhanced toughness, thermoplastic-like ductility, and thermally-induced shape-memory recovery (see Figure 1) that the authors attributed to the non-covalent interactions of rigid triazole-rings and to a sufficiently high bond rotational freedom between network junctions. Key merits also include low resin viscosity, ‘bulk’ photo-polymerization, rapid photo-curing kinetics, and formation of homogeneous network structure with readily tunable backbone functionalities. Remarkably, (Cu-free) photo-initiated thiol-ene polymerization of triazole-based monomers offers the additional advantage of yielding transparent, non-toxic thermosetting polymer films.
By providing efficient synthetic protocols for monomer design, rapid in situ photo-curing kinetics, and extremely high-toughness glassy polymer networks, Bowman et al. offers a potential solution to the current demand for photo-curable glassy thermosets with high extensibility (comparable to conventional tough thermoplastics), and further provide foundations toward emerging photopolymer applications such as additive manufacturing technology.