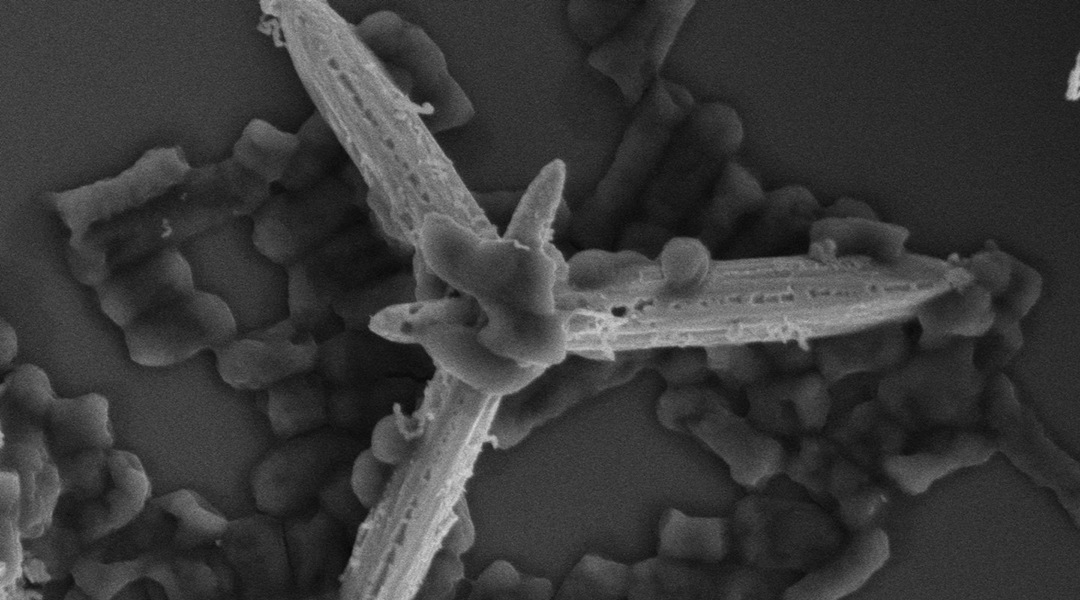
Worldwide, nearly five million deaths each year are associated with antimicrobial resistance. Developing newer, stronger antimicrobial medications is one approach for combating the problem, but it’s always better if you can keep harmful microbes from making people sick in the first place.
In a new study published in Advanced Optical Materials, researchers from the Institute of Chemical Research of Catalonia in Tarragona, Spain have proposed a unique mechanism for destroying microbes in food and water systems: photoactive micromotors.
“A photoactive micromotor is a tiny artificial machine that can move around in a liquid, just like tiny living organisms do,” said Katherine Villa, who co-authored the research. “But instead of relying on biological processes, these micromotors are powered by light-induced chemical reactions that trigger their autonomous motion.”
As they move, Villa said, the micromotors produce highly oxidative radicals and release silver ions and nanoparticles. “Both the silver and the reactive radicals are effective at damaging the cell membrane of bacteria, ultimately causing their death. It’s like they’re a double threat to bacteria, attacking them from multiple angles and ensuring their destruction.”
When the researchers tested the micromotors against the common illness-causing bacteria E.coli and S.aureus, they were 99.999% efficient at destroying them.
Self-degradation improves the kill-rate
Part of the micromotors’ effectiveness comes from their ability to self-degrade. As the micromotors release silver ions, they gradually begin to break down. In the presence of bacteria, the self-destruction happens even faster, with the micromotors breaking down completely in less than one minute.
“Consequently, there is an increased dispersion of silver nanoparticles in the surrounding liquid, enhancing their contact with bacteria and resulting in heightened antibacterial activity,” said Villa.
The self-destruction also ensures that the micromotors don’t remain in the environment. “Instead, they break down into harmless components over time, reducing potential environmental impact,” said Villa. “Overall, self-degradation plays a crucial role in maximizing the efficacy of the micromotors for bacterial removal while ensuring environmental safety and sustainability.”
Real-world applications
In practical use — for example, to destroy pathogenic bacteria in a meat packing plant — Villa said the micromotors could be added to a cleaning solution, which would be applied to surfaces and equipment. The cleaning solution could then be activated with light. “Similarly, in contaminated water sources, these micromotors could be introduced to disperse and move around, actively neutralizing harmful bacteria while minimizing environmental impact.”
“They applied [micromotors] to both free-swimming bacteria, which are kind of easy ones to target, but also biofilms,” said Craig Stephens, professor of biology and public health at Santa Clara University, who was not involved in the study. A biofilm is a community of microbes protected by a slimy, extracellular layer, which stick together on surfaces.
Biofilms are notoriously difficult to eradicate since they can become resistant to antimicrobial agents. In their paper, the researchers demonstrated decreased S. aureus-based biofilm viability of up to 93% in the presence of micromotors and light. In theory, Stephens said, “this might be an approach to removing biofilms from difficult-to-access environments more effectively and possibly without quite as harsh chemical treatments.”
Next steps
Stephens said one of the challenges will be going from basic science to real world uses. “The question is, what sort of applications will they be able to find where this is really advantageous over existing technologies? What’s the sweet spot, whether it’s cleaning difficult to deal with surfaces in healthcare environments or water treatment applications, water distribution, [or] system applications,” said Stephens.
“There are definitely contexts in healthcare settings, whether it’s hospitals or long-term care facilities where it can be really hard to remove some tough biofilm-forming pathogens so that they don’t get transmitted to somebody else. So it’s intriguing to me whether this could be relevant to situations like that.”
There are also still questions that need to be answered about how micromotors destroy bacteria and what effects they might have after they’ve done their work. For example, Villa said the researchers still don’t know why the micromotors break down faster in the presence of bacteria.
“The next steps in our research entail studying the mechanisms of interaction between the micromotors and bacteria,” she said. “Additionally, we aim to further evaluate the environmental impact of the micromotors after degradation.”
Once these questions are answered, micromotors may join the arsenal of strategies scientists and healthcare providers have for combating the growing problem of antimicrobial resistance. With a little more research and development, micromotors may even prove to be a life-saving technology.
Reference: Xiaojiao Yuan, Katherine Villa, et al., Self-degradable Photoactive Micromotors for Inactivation of Resistant Bacteria, Advanced Optical Materials (2024). DOI: 10.1002/adom.202303137