Image credit: Magnus Biggs/Marc Fernández Yagüe
Although tendon injuries may appear to happen suddenly, they are often the result of continuous stress or wear and tear that occurred over time. These injuries are common among athletes — with the internet going as far as providing a “10 Famous Achilles Tendon Ruptures” list for the bored browser to peruse — but the burden on the healthcare system is not trivial, with over 100 million adults affected every year.
Considered the gold standard in treatment, surgery boasts relative success. However, it frequently fails to restore full tendon functionality as a result of scar tissue formation.
“Scar tissue, and in particular calcification of the tendon following injury, can develop from aberrant inflammatory processes,” explained Manus Biggs, professor at SFI Research Centre for Medical Devices based at NUI Galway. “This scar tissue is non-functional, mechanically inferior to the natural tendon, and can lead to chronic pain, stiffness, and joint instability.”
In a recent study published in Advanced Materials, Biggs and a collaborative team of researchers say that meaningful, long-term repair for these types of injuries needs to involve activation of the body’s tissue-repair pathways. To do this, they turned to electrical therapy, coupled with exercise, using an implantable device that is powered by movement.
“Here, we explored fundamental processes of basic cell biology and how these can be translated into clinical solutions,” said Professor Abhay Pandit, one of the study’s authors and fellow researcher at NUI. “Identifying how specific cell membrane receptors respond to electromechanical stimulation is a really exciting development in the design of next-generation regeneration strategies.”
Prior studies have shown the potential of electrical fields in assisting wound healing by mediating cell migration and promoting the synthesis of collagen proteins, which are known to be beneficial to the healing process. Collagen, like DNA and bone, is a natural piezoelectric material — a category of materials that can generate an electrical charge when mechanical stress is applied to them. This has spurred new research into the role of bioelectricity in tissue regeneration and the development of self-powered electrical stimulators which provide biomimetic electric fields.
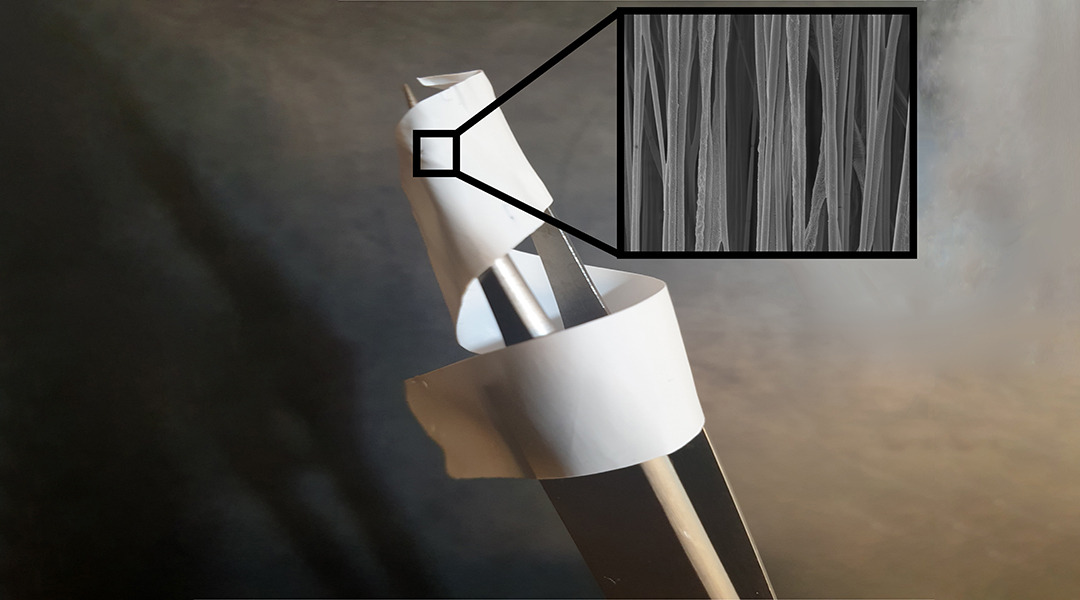
The team, therefore, built an implantable device that acts as mechanical support for damaged tendon tissues and mimics the bioelectrical cues usually provided by collagen. “It can be used as a strong, suture-like material, or more practically, as a conduit-type material, which wraps around a tendon after it has been sutured by a surgeon performing a repair,” said Biggs.
“As well as assessing the biological response to these piezoelectric materials, it was critical that we gain an understanding of how a piezoelectric polymer can be formulated into microscale fibers,” explained Matteo Palma, who contributed materials expertise to the study. “We used cutting-edge scanning probe microscopy to assess the morphological and piezoelectrical properties of these fibers, and how these properties change when fibers are formed into an extensive meshwork.”
Their device uses a synthetic, mesh-like fabric made from a ferroelectric polymer — a material that once an electric field or mechanical stretch is applied, possess a permanent polarization Critically, this material was organized into aligned microfibres, behaving as a collagen analogue.
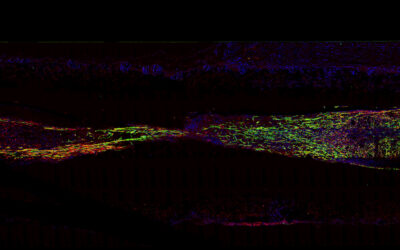
“Our discovery shows that an electrical charge is produced in the treatment target area — the damaged or injured tendon — when the implanted device is stretched during walking,” said lead author Marc Fernández Yagüe. “The potential game-changer here is like a power switch in a cell — the electrical stimulus turns on tendon-specific regenerative processes in the damaged tendon.”
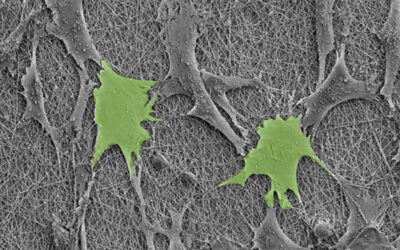
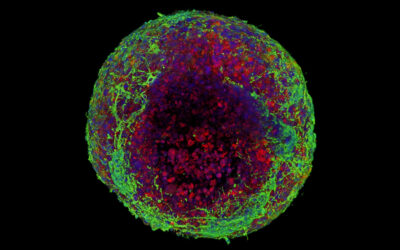
In in vitro experiments, the researchers showed that the motion-powered stimulation of tendon tissue through their piezo-bioelectric device activated ion channels in tendon cells that helped regulate specific tissue regeneration signaling pathways.
“One of the most exciting parts of our study is that these implantable devices may be tailored to individual patients or disorders and may show promise in accelerating the repair of sport-related tendon injuries, particularly in athletes,” said Biggs in a statement. Since the device is compliant and flexible, it can be easily cut and fitted according to a patient’s needs and degree of injury.
“These devices are cost-effective, relatively easy to implant and may pave the way for a whole new class of regenerative electrical therapies,” he continued. “This unique strategy of combining a device which is powered through body movement, and which can induce accelerated tendon healing is expected to significantly impact the field of regenerative devices, specifically in the area of sports or trauma associated injuries.
Reference: Marc Fernandez-Yague, et al., A Self-Powered Piezo-Bioelectric Device Regulates Tendon Repair-Associated Signaling Pathways through Modulation of Mechanosensitive Ion Channels, Advanced Materials (2021). DOI: 10.1002/adma.202008788