With the rapid development of life science and pathology, research on intracellular active species, cell signal transduction, and apoptosis by fluorescen ce microscopy imaging has become an important and active field. For biological samples, the target fluorescent signal often suffers from interference with background fluorescence, reducing the target-to-background ratio. An effective strategy to address this problem is to utilize a time-gated technique, which requires long-lived phosphorescence as the target signal and can effectively eliminate the interference from short-lived background fluorescence.
ce microscopy imaging has become an important and active field. For biological samples, the target fluorescent signal often suffers from interference with background fluorescence, reducing the target-to-background ratio. An effective strategy to address this problem is to utilize a time-gated technique, which requires long-lived phosphorescence as the target signal and can effectively eliminate the interference from short-lived background fluorescence.
Although many phosphorescent probes have been developed for sensing various analytes, most of them suffer from poor water-solubility. The addition of organic solvents is often needed in sensing and bioimaging, limiting their real biomedical applications. Hence, there is an urgent need to develop effective strategies to exploit water-soluble and biocompatible phosphorescent probes.
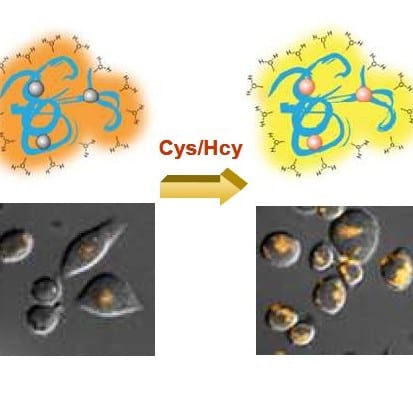
The group of Zhao and Huang (Nanjing University of Posts & Telecommunications, Nanjing, China) reports now a versatile strategy to exploit water-soluble and biocompatible polymer phosphorescent probes by introducing an iridium(III) complex as a signaling unit into a stimuli-responsive poly(N-isopropylacrylamide) backbone. This bioprobe can be used for sensing homocysteine and cysteine – two important amino acids in the human body – in aqueous systems through the variations in absorption and photoluminescence spectra. The probe is thermo-responsive and, more importantly, exhibits low cytotoxicity. It has been successfully used for live-cell imaging demonstrating that the probe is membrane permeable.
The combination of excellent photophysical properties and very low cytotoxicity renders this material a promising probe for bioimaging applications.