Many disease treatments aim to inhibit or block a protein’s function somewhere in the body. A great way of doing it is by impeding protein production using molecules called small interference RNA, or siRNA for short.
“siRNA-based therapeutics are poised to revolutionize treatment options for a variety of diseases ranging from autoimmune diseases to cancer,” said Lichen Yin, professor at the Institute of Functional Nano and Soft Materials at Soochow University in China.
Proteins are made in a process called translation from messenger RNA (mRNA). siRNA molecules are unique in that they “stick” to mRNA molecules, hiding them from the cell’s machinery when necessary, and thus allowing them to decrease translation.
To date, there are just five siRNA-based therapies approved to treat rare metabolic diseases. While potent therapeutics, nucleic acids — the molecules that make up siRNAs — are easily degradable, making them difficult to deliver, but a way around this is to coat them in protective carrier.
Evading degradation
Carriers developed to date can be used to deliver siRNA only when the trip is short and direct. This is accomplished via injections to avoid the body’s natural barriers, such as the stomach and GI tract, which destroy them or physically impede their arrival to target organs.
But injections are not practical for patients, says Yin. Some require medical professionals to perform them safely, or even when patients can do them themselves, they can be painful and carry a risk of infection. A much easier way for a therapy to be delivered is by oral intake. “Oral delivery is convenient and non-invasive, typically favorable for people suffering from chronic diseases and requiring frequent dosing,” said Yin.
However, since most siRNA carriers are not built to withstand the harsh conditions found in the gut, Yin and his research team needed to develop a tougher carrier to protect siRNA, and this way provide patients with an oral, easy, and practical means of delivery.
A protective siRNA vehicle to traverse the stomach
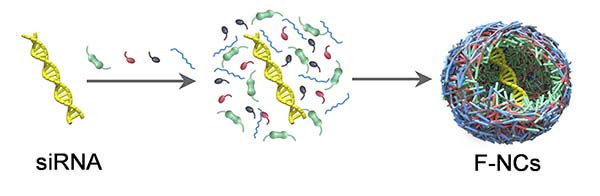
In their recent study published in Advanced Materials, the team showed the development of fluorinated nanocarriers that protect nucleic acids’ passage through the gut.
“This study develops a robust oral delivery technology for siRNA drugs, assisted with a small-sized, fluorinated nanocapsule that features high stability during transit in the gastrointestinal tract, high absorption in the small intestine, and high systemic gene silencing efficiency,” said Yin.
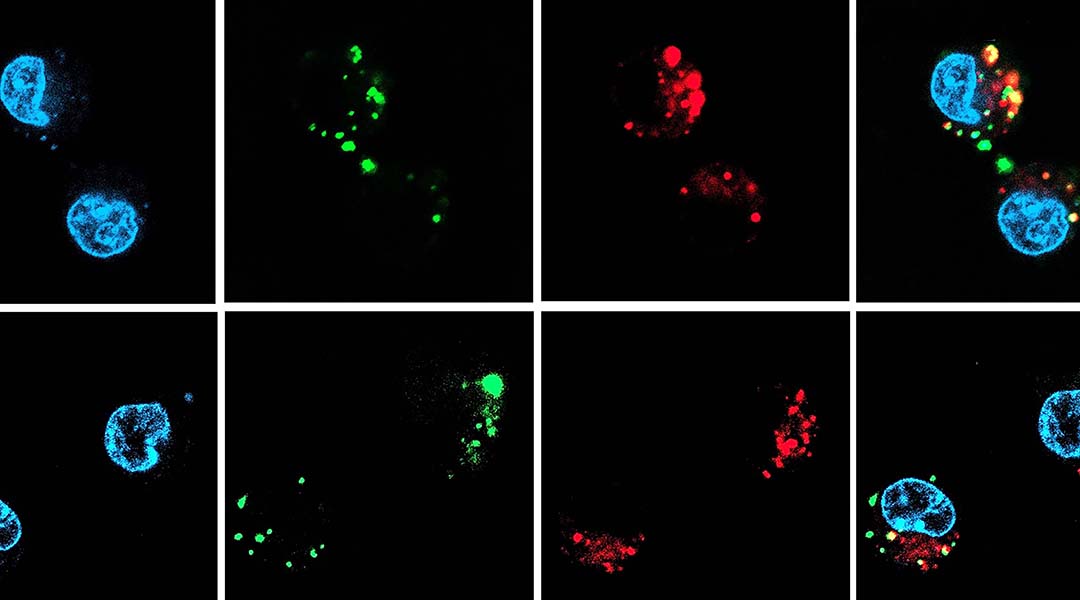
To study the ability of the carried nucleic acid in blocking protein production, the team chose an siRNA that could treat inflammatory diseases by blocking the production of a signaling protein called tumor necrosis factor alpha, or TNFα for short — a known target to treat inflammation.
The acidic medium in the stomach poses the greatest challenge to this class of drug. They therefore need a resistant shell to protect the drug payload.
The team started by encapsulating the TNFα siRNA molecules into nanocapsules shells, building them through a reaction involving in situ free radical polymerization and then tested their acidic resistance. The scientists put the nanocapsules in an acidic suspension at the same pH level as the stomach: 1.2. They later changed it to pH 6.8 to match that of the intestinal tract. After both acid/base treatments, the team observed that the nanocapsules were unaffected and the siRNA was still encapsulated inside its carrier.
But the carrier needs to release the siRNA once it enters the cell to carry out its therapeutic role. To make their nanocarriers breakable inside cells, the team added a particular molecule to the shell that is cleavable in the presence of a glutathione, a compound normally found in high concentrations within cells’ cytoplasm.
To test it, the scientists incubated the nanocapsules containing the TNFα siRNA at two glutathione concentrations: one normally found outside the cells and a higher one found in the cell cytoplasm. After 24 hours of treatment they observed what they had been hoping for — that the nucleic acid molecules were only released when in presence of cytoplasmic glutathione concentrations.
Testing the nanocarrier as an anti-inflammatory therapy
An issue with these types of nanocarriers is that they tend to get stuck in the mucus layer of the intestines. This is because for one, the positive charges normally present on the carriers generate electrostatic interactions with proteins of the mucus layer which immobilizes them. Secondly, the carriers get stuck in the physical barrier formed by a protein mesh in the mucus with spacings of about 100 nm. This produces a generally low permeability through the intestine, preventing the siRNA drugs from getting into the bloodstream and reaching cells in target organs.
The nanocapsules the team developed have two important components that make them different to previous carriers. They incorporate a fluorocarboned polymer which avoids electrotactic attraction with proteins in the mucus, and they were built small, with sizes of about 30 nm, allowing them to pass through the protein mesh.
“Normally, it is not easy to prepare polymeric nanocarriers for such small sizes,” said Yin. “For nucleic acid delivery, a common approach is using polycationic materials, which can self-assemble with siRNA via electrostatic interactions, forming nanocomplexes with sizes around or above 100 nm. In this study, we used the in situ interfacial polymerization technique. That is, monomers polymerize and crosslink on the surface of siRNA molecules, simultaneously encapsulating siRNA inside the polymeric shell. Using this technique, small nanocapsules with diameter < 30 nm could be obtained.”
To test their nanocarriers permeability, the team gave them orally to mice and followed their distribution, finding they passed through the mucus layer of the intestine and reached the circulation in acceptable quantities.
“The nanocapsules can be directly absorbed into blood circulation and then distributed into different organs, and this may benefit the wide application for the treatment of [many] diseases,” said Yin.
Treated mice had a decreased level of the inflammation protein in circulating blood and of mRNA in the liver, showing the siRNA can reach and perform its function in other organs. Moreover, treated mice had a better survival rate than mice treated with non-fluorinated nanocarriers, validating the therapy as an effecting way of treating inflammation.
While the principal purpose of this study was to develop carriers resistant to the gut, and the authors now plan to expand its application.
“This technology can be applied to different siRNA drugs or even mRNA drugs for the treatment of various human diseases, and we expect it to find more useful utilities such as mucosal immunization and genome editing,” said Yin.
Reference: Yuansong Wei, et al., Oral Delivery of siRNA Using Fluorinated, Small-Sized Nanocapsules toward Anti-Inflammation Treatment, Advanced Materials (2023). DOI: 10.1002/adma.202206821
Feature image: Characterization of nancapsules and intracellular TNF-α siRNA delivery efficiency in macrophages in vitro.