At the University of Utah, Mohassab and Sohn are leading an investigation on the chemistry of slag under the conditions of a transformational ironmaking technology being developed there. The project is intended to develop an entirely novel process for alternate ironmaking based on the direct gaseous reduction of iron oxide concentrates in a flash reduction process, with the ultimate objective of significantly reducing energy consumption by up to 57% and reducing environmental emissions, especially CO2 emissions, compared with the conventional blast furnace ironmaking route. The proposed system, which the researchers call flash ironmaking technology, uses gaseous reducing agents, such as natural gas, hydrogen, other syngas, or a combination thereof. The proposed technology could be applied to the production of iron as a feed to the steelmaking process, eventually replacing the blast furnace and other alternate ironmaking processes. It can also be used as part of a continuous direct steelmaking process.
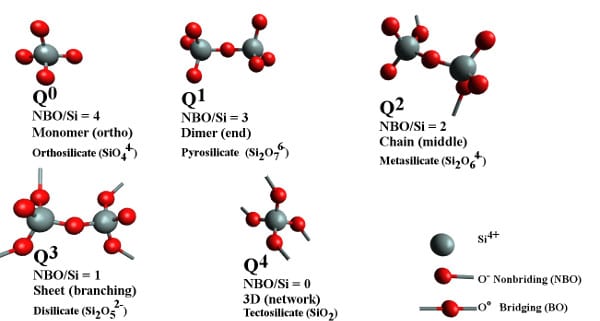
Models of silicate melt structural entities that coexist in different ratios depending on the chemical environment. Q-notation for each structural unit and non-bridging oxygen (red) per silicon atom (grey), NBO/Si, are two different ways to indicate the extent of polymerization. The term bridging oxygen describes an oxygen atom boding two silicon atoms together.
Mohassab and Sohn have determined that water stabilizes the more polymerized silicates anions rather than the depolymerized monomers. They concluded that the higher the water content in the gas atmosphere the more polymerized the silicates in the slag. This difference in polymerization degrees plays a critical role in the distribution of elements between slag and molten iron as well as the activity coefficients of oxides in the slag. In addition, the degree of polymerization controls the physical properties of the slag such as the viscosity. Based on the Fourier Transform Infrared and Raman spectroscopic analyses, they discovered that H2O in the gas atmosphere increases the degree of polymerization of silicate anions in the slag and accordingly the slag viscosity. The researchers belief that their work provides a comprehensive and comparative analysis of the effect of H2O in the gas on the chemistry of an ironmaking slag.