Hydrogels can mimic many aspects of living tissue and as such can act as synthetic surrogates for mammalian cells by providing a hydrated environment with certain mechanical and biological characteristics. Hydrogels are formed when macromolecules (polymers) that can dissolve in water are linked together to form a fishnet-like massive network that can absorb and hold water. The nature of the connection points between the macromolecules defines much of the physical properties of the hydrogel. Increasing the number and/or strength of the connection points (crosslinks) will make the hydrogel stiffer and vice versa. However, controlling these connection points to produce hydrogels of defined properties for use within the human body is challenging as the chemistry required for the formation of the connection point is not always easy to implement within the environment of the human body and also may pose toxicity issues.
In the cover article of the August 6th issue of the Proceedings of the National Academy of Sciences, USA, Forget et al., present an intriguing strategy to produced hydrogels of precise stiffness and demonstrate how such hydrogels can be used to control the organization of blood vessel cells into vascular structures. Polymers typically have a random structure in solution like a bowl of spaghetti. However, poly(sugars) (polysaccharides) like agarose that is sourced from sea algae and carrageenan have spring-like structure and they can form hydrogels by the association of these spring-like motifs into connection points. The research team discovered that by modifying the polysaccharide backbone they could switch the structure from spring-like (helix) to sheet-like (ribbons).
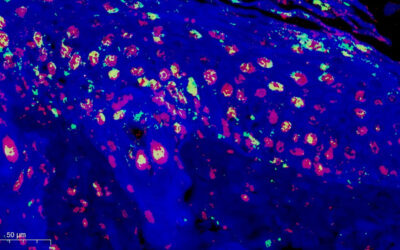
By adopting ribbon structures the polysaccharide forms weaker connections and by varying the amount of modification it is possible to control the ratio of ribbon motifs and thus hydrogels with precise stiffness to match every tissue environment in the human body can be realized. As the stiffness of the material surrounding a cell (i.e., extra cellular matrix) influences how that cell behaves, control over cell function maybe exerted by varying the stiffness around a cell, which is the cornerstone of a new paradigm called mechanobiology. By screening hydrogels containing varying amount of ribbon-like structures i.e., mechanical environments, in combination with molecules that maintain function of blood vessel cells (endothelial cells), the group were able to demonstrate that mechanobiology plays a very critical role in the organization of endothelial cells.
They discovered, that while stiff gels fail to induce organization of endothelial cells, softer gels, which resemble the environment associated with an embryo, could impose changes to cell function that promotes the organization of endothelial cells into freestanding blood vessel-like structures. The mechanically tunable hydrogels are expected to have a significant impact in the development of therapies for regenerative diseases, where mechanobiology is used as a tool to control cells and their organization into functional tissues through minimally invasive strategies.