The material NVP (Na3V2(PO4)3) has attracted widespread attention for use as a cathode material in sodium ion batteries. This material has a NASICON framework suitable for fast Na+ transport, but its wide application in sodium ion batteries is limited by the poor intrinsic electric conductivity, which results in poor electrochemical performance. Many approaches have been reported to increase the ion and electron transport kinetics in batteries including enhancing the electronic conductivity by coating a conductive surface layer and tailoring the particle size (or morphology) of the electroactive materials. Constructing low dimensional electroactive materials has been demonstrated to be a very effective way to improve the electrochemical performance due to the drastically shortened transport distance for both ions and electrons. Among these, one dimensional (1D) materials show unique characteristics, including high surface to volume ratio, short transport lengths for ionic transport and efficient 1D electron transport along the longitudinal direction.
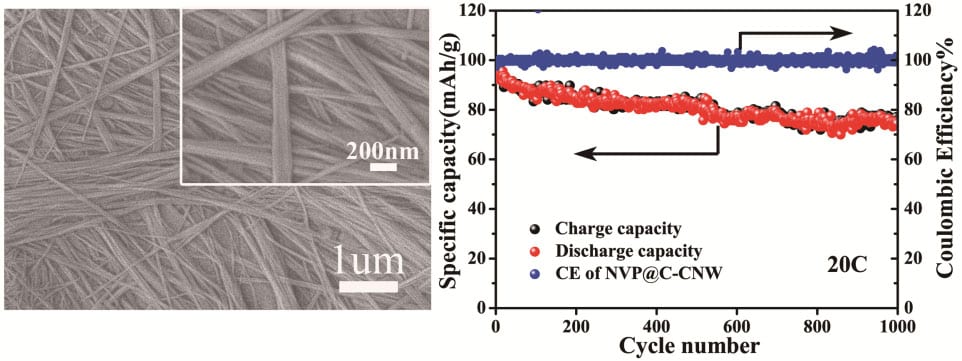
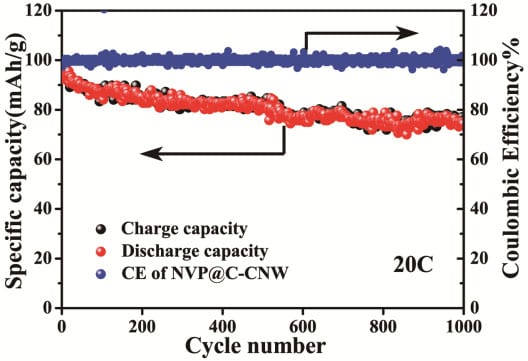
Recently, Prof. Yan Yu’s group at the University of Science and Technology of China developed a facile hydrothermal method to synthesize carbon-coated NVP embedded in 1D porous carbon nanowires (NVP@C-CNW) for high performance room-temperature Na-ion batteries. This special structure has a variety of advantages. First, the porous 1D nanowire structure could not only shorten the sodium ion diffusion pathways but also offer enough space for the volume expansion of the active material during repeated charge/discharge cycles. Second, the large surface area could make it easy for the electrolyte to soak in, which greatly enhances the sodium ion diffusion rate. Finally, the core-shell structure could effectively restrain the particle growth during the heating process and enhance the electric conductivity. When used as cathode for Na-ion batteries, they display long cycle life (only 9.6 % capacity loss after 1000 cycles at 1 C with a capacity retention of 96.6 mA h g-1) and an outstanding rate performance (a reversible capability of 84.2 mA h g-1, 77.0 mA h g-1 and 62.2 mA h g-1 at 30 C, 40 C and 60 C, respectively). The synthetic protocol could be further extended to prepare other anode and cathode materials for both lithium-ion batteries and sodium-ion batteries with high power and energy densities.
This paper is part of a special issue on Nanomaterials for Energy Conversion and Storage in ChemNanoMat.
Text kindly provided by the authors of the original manuscript.