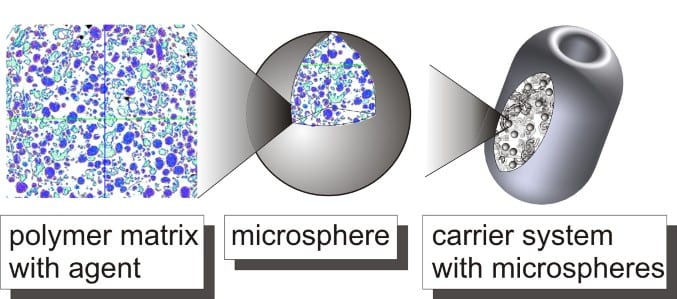
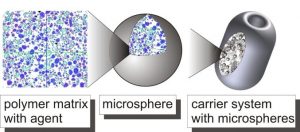
The Institute of Plastics Processing (IKV) at RWTH Aachen University is investigating, together with partner institutes, the controlled release of drugs for the treatment of common diseases of the urinary bladder such as, for example, the condition known as overactive bladder. The drug delivery system (DDS) consists of a drug-loaded polymer matrix – so-called microspheres – that are embedded in a foamed absorbable carrier system. Within this joint project, it is the IKV’s task to develop the foamed carrier system. Other project partners are Dr. Pfleger GmbH, Hemoteq AG, DWI at RWTH Aachen, and the department of Urology of the University Hospital Aachen (UKA).
In this novel treatment approach the drug delivery system (DDS) is directly placed into the urinary bladder. The drug release and the excretion of the system are controlled by the degradation of the carrier system. Thus, the active agent has a locally lasting effect in the bladder and won’t affect the whole body, in contrast with the intake of tablets. Also regular catheterisation, often several times a day, is no longer required.
The carrier system is manufactured at IKV using the CESP process (Controlled Expansion of Saturated Polymers). The technology of the CESP process enables the possibility of processing temperature-sensitive materials, like the used poly (D,L-lactide-co-glycolide)-co-PEG. The material can be processed in a CO2 atmosphere at high pressure (approx. 50 bar) at low temperatures of approx. 50 °C. By an extension of the CESP process, a powdery polymer microsphere mixture can be foamed specific via a pressure controlled, continuous variable discharge. The adjustment of the degradation of the carrier system to medically necessary periods is possible by the termination of the foam structure. For reproducible manufacturing of the carrier system in the range of micrograms a dosing unit and adapted cavities are integrated into the process chain.
This extension of the CESP process allows more applications in the scope of absorbable, drug-eluting implants. By means of the reproducible dosing of the materials and the optimised controlling of the process, porous osteosynthesis plates or stents are as well possible.
Source: IKV Aachen