Metals that are liquid at room temperature are unique because they have metallic electrical properties, but can flow like liquids, and can therefore be utilized in ways which would be impossible with solid metals. The ability to control the shape of liquid metal is crucial for a variety of applications, including soft electrodes, microfluidic components, soft robotics, sensors, stretchable wires and antennas, and self-healing circuits.
There are a number of ways to pattern these liquid metals, but most are complex. Michael Dickey and colleagues at the North Carolina State University have found a very simple way to pattern liquid metals, requiring only a battery, salt water, and two wires.
In their article in Advanced Materials Interfaces the group describe locally reducing the surface oxide that forms spontaneously on the liquid metal eutectic gallium indium, which induces capillary withdrawal of the metal away from the counter electrode. They call this effect recapillarity because of the use of electrochemical reduction to induce capillary action.
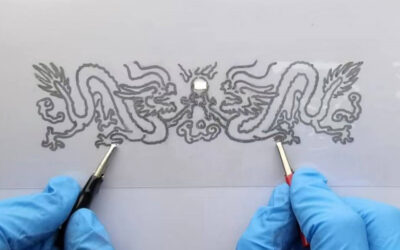
 The technique is based on the use of instabilities in the film of liquid metal, for example pinholes. The instabilities are locally induced in the film by electrochemically removing the oxide, using an electrode as a “scribe”, that is, a copper wire dipped in electrolyte, to pattern the metal (see image). Unlike conventional scribes, which physically scratch away material, their “scribe” never touches the film, but instead localizes electrochemical reactions that induce localized instabilities. Upon removal of the oxide from the surface, the exposed metal returns to a state of high interfacial energy and withdraws locally and spontaneously to minimize its interfacial energy, but stops flowing in the absence of a reducing potential due to the spontaneous reformation of the oxide.
The technique is based on the use of instabilities in the film of liquid metal, for example pinholes. The instabilities are locally induced in the film by electrochemically removing the oxide, using an electrode as a “scribe”, that is, a copper wire dipped in electrolyte, to pattern the metal (see image). Unlike conventional scribes, which physically scratch away material, their “scribe” never touches the film, but instead localizes electrochemical reactions that induce localized instabilities. Upon removal of the oxide from the surface, the exposed metal returns to a state of high interfacial energy and withdraws locally and spontaneously to minimize its interfacial energy, but stops flowing in the absence of a reducing potential due to the spontaneous reformation of the oxide.
The method does not provide the precision or resolution of some other patterning methods, but its unrivalled simplicity makes it particularly accessible.