With their applications extending into large-scale stationary storage fields including transportation and grid, traditional Li-ion batteries (LIBs) based on layered Li-intercalation electrode materials are limited by the intrinsically low theoretical capacities of both electrodes, and could not meet the increasing demand on energy. Against this background, nanostructured anode materials based on novel Li storage mechanisms, such as conversion storage and interfacial storage, are especially promising in constructing the next generation high-energy LIBs due to their predominant merits in capacity. However, combating issues, such as sluggish kinetics of electrode reaction and poor structural stability upon cycling, still hinder their practical applications.
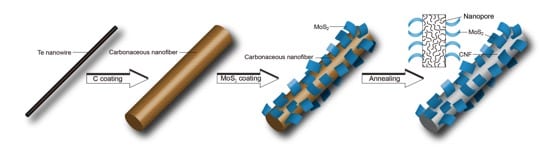
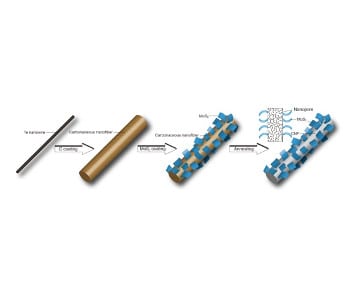
Fig. 1 Synthetic procedure for CNFs@MoS2.
To address the above problems, Shu-Hong Yu and his team at the Hefei National Laboratory for Physical Sciences at Micrscale (HFNL), University of Science and Technology of China (USTC) have recently developed a facile way to construct “kinetically stable” anode materials for high-energy applications. They elaborately selected two study objects, one is molybdenum disulfide (MoS2), a dichalcogenide anode material promising for its ability to host multiple Li ions via an “insertion-conversion” process, leading to significantly higher theoretical capacity than intercalation anodes, such as TiO2 or TiS2. The other is porous carbon, which is also a strong candidate for anode application in LIBs by taking its advantages of a large specific surface area and a hierarchically porous structure, enabling unique interfacial and/or nanopore Li storage processes and bringing specific capacities several times larger than that of graphite. Both materials are appealing choices in building high-energy LIBs, yet their defects are also obvious: MoS2 is known to have a layered structure formed by tightly packed S-Mo-S, which enables a stable Li insertion at > 1.1 V vs. Li+/Li yet converts to Mo and Li2S upon further Li intercalation. Owing to a low conductivity and poor cyclability of Li2S, the capacity of MoS2 often fades quickly upon cycling. Moreover, with a significant volume change during discharge/charge, MoS2 anode usually experiences pulverizations, resulting in poor cycling performance. On the other hand, porous nanocarbon materials usually have excessive interface with electrolyte, which contribute to considerable side reactions, resulting in poor cyclability and low Coulombic efficiency. Given a combination of the two material may contribute to a synergistic effect to overcome the above deficiencies, Shu-Hong Yu and his team proposed and synthesized a new nanocomposite, in which MoS2 sheets were decorated on carbon nanofibres (CNFs@MoS2) via a facile hydrothermal route with low-cost, biomass-derived, carbonaceous nanofibres as supports.
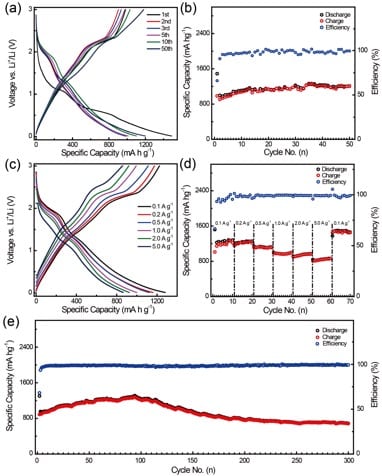
Fig. 2 (a) GDC profiles and (b) cycling performance of CNFs@MoS2 at 0.1 A g-1. (c) GDC profiles and (d) cycling performance of CNFs@MoS2 at different current densities. (e) Long-life cycling performance of CNFs@MoS2 at 1 A g-1.
The cooperation of the two active components brings a favorable synergistic effect, delivering much improved electrochemical performance compared with those delivered by any of the single component. Moreover, it also triggers a new electrochemistry on Li storage, by which a persistent transformation from Mo (or MoS2) to MoS3 with larger capacity and better cyclability is enabled during the repeated charge processes, resulting in a continuous increase in composite capacity upon cycling. The CNFs@MoS2 nanofibers exhibit excellent Li storage properties in terms of specific capacity (1489 mA h g-1 upon initial discharge), cycling performance (1264 mA h g-1 after 50 cycles) and rate performance (860 mA h g-1 at 5 A g-1), making it a promising anode material for high energy LIBs. Moreover, in the light of the notable capacity advantages of non-intercalation materials in high-energy batteries, this pioneering work in finding the synergistic effect of CNFs@MoS2 is expected to have far-reaching significance over previous works on intercalation materials. The science behind the strategy is simple yet inspiring, and because of its versatility, could also bring insights to those working on high-energy batteries, benefiting the industry and contributing to a better economic sustainability. The results have been recently published in Angew. Chem. Int. Ed.
The research project is sponsored by the National Basic Research Program of China, the National Natural Science Foundation of China and the Chinese Academy of Sciences.