Despite the availability of antifungal therapies, the World Health Organization (WHO) considers fungal pathogens a serious threat to public health. Candida albicans, for example, is a common yeast fungus that causes localized infections, such as oral thrush, and has the potential to become invasive, even fatal.
Current therapies lack speedy and targeted action and can lead to various side effects. Moreover, the landscape of antifungal treatment is plagued by emerging drug resistance. As a result, people who are unresponsive to first-line antifungals may need extensive and costly second-line treatments.
Hyun (Michel) Koo and Edward Steager, researchers at the University of Pennsylvania, brought together their teams in a collaborative effort to use microrobots to target microbial films on soft surfaces.
“Our study has created a new approach using tiny robotic particles called nanozyme microbots to fight fungal infections caused by Candida,” said Koo, in an email to ASN. “These nanozyme-bots can be controlled to precisely target the infection site and rapidly eliminate fungal pathogens.”
Nanoscale particles with catalytic and magnetic properties, called nanozymes, have shown some promise in treating bacterial infections. But as their application as antifungals has been limited, Koo and Steager chose to direct these nanozymes to tackling this challenge.
How nanozymes kill fungal cells
Nanozymes made of iron oxide particles offer dual advantages: they can be manipulated by magnetism and their catalytic capability can help kill microbes. Like the enzyme peroxidase found in our body, iron oxide nanoparticles can kick start a reaction that breaks down hydrogen peroxide into more destructive forms of oxygen known as reactive oxygen species, which kill fungal cells.
To begin with, the research team used a simple and low-cost chemical reaction to create iron oxide particles that made up the nanozyme microrobots. Using an externally applied electromagnetic field, the researchers maneuvered the movement of the nanozymes to vibrate, roll, glide, or dab, and move to a specific target.
The form and motion of the nanozyme guides it to a particular spot and influenced its catalytic ability at the target site. For instance, vibration and dabbing motions were most conducive to targeted delivery. On the other hand, rolling and gliding best suited the nanozyme’s potential to break down hydrogen peroxide.
Once they established the nanozyme’s range of motions, the team attempted to kill C. albicans using these microrobots. The robots along with hydrogen peroxide and fungal cells were brought into contact within a chamber. Within 10 minutes, the nanozyme robots catalyzed the production of reactive oxygen species and killed off all C. albicans.
“To precisely and reproducibly treat the biofilm with nanozyme microbots, we created a magnetic field control device and programmable algorithms that automates the targeting process developed in our laboratory,” said Koo. The direction of the magnetic field, and thus, the motion of the nanozymes, was maintained by pre-programmed motorized devices that were in perpetual motion.
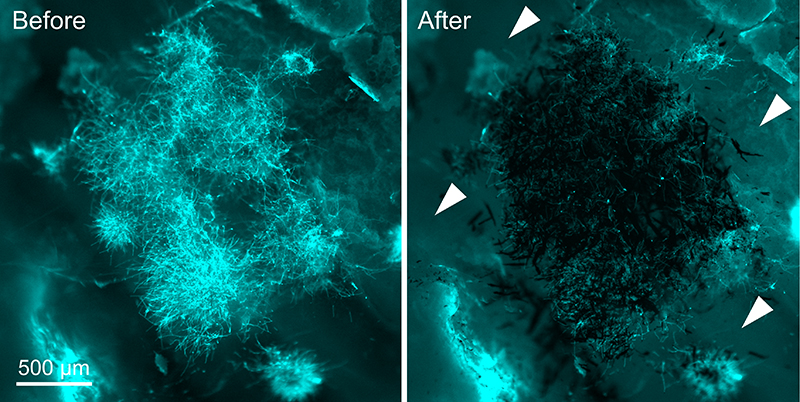
In order to analyze the nanozyme’s binding specificity and killing ability in a more realistic setting, the researchers tested it using a clump or spheroid made of human gum cells. The cell spheroids of gum tissue were mixed with C. albicans cells to mimic a fungal infection.
Using magnetism, the researchers targeted the nanozyme microrobots to latch onto the fungal cells while avoiding the gum cells. Once captured, the nanozyme-fungal complexes were moved into a small chamber, where the killing ability of the microrobots was assessed.
In the presence of hydrogen peroxide, the nanozymes successfully stimulated the production of a large amount of reactive oxygen species that killed all fungi on-site within 10 minutes.
A preference for fungus
The researchers made an unexpected observation: the nanozymes had a strong affinity for fungal cell surfaces — a binding preference that might be crucial to targeted delivery.
“We were surprised to find that these nanozyme assemblies strongly adhered to the fungal cells, particularly when compared to human cells,” said Koo. “This feature enables a localized accumulation of nanozymes precisely where the fungi reside and, consequently, targeted antifungal generation and rapid eradication of fungal cells without binding to human tissue.”
To study the infection in a true-to-life anatomical setting, the researchers created an experimental model of the mucosal membrane of the murine mouth infected by C. albicans. Once again, the microrobots were found to selectively bind fungal cells, resulting in a concentrated delivery of reactive oxygen species where needed, minimizing damage to healthy host cells.
More research is needed to understand how these microrobots function. For instance, the researchers found tracking the real-time location of reactive oxygen species, a measure of the nanozymes’ killing ability, especially difficult.
In the future, the team want to better understand the mechanisms underlying the specific binding between nanozyme microrobots and C. albicans cells. A better understanding of the binding dynamics may offer some insight into whether the nanozymes could target other microorganisms. Koo’s team would also like to test and validate the nanozymes using animal and clinical models.
Despite some unknowns, these microrobots seemingly offer a speedy, targeted, and affordable way to wipe out infections caused by C. albicans. “Our findings open doors for developing better antifungal therapies using nanozymes that could be more effective, reduce the development of drug resistance, and improve drug delivery without side effects compared to current methods,” said Koo.
Reference: Edward Steager, Hyun Koo, et al., Nanozyme-based robotics approach for targeting fungal infection, Advanced Materials (2023). DOI: 10.1002/adma.202300320