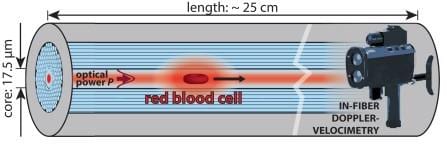
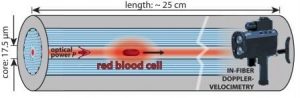
A new tool enables biomechanical studies of individual cells: Red blood cells were laser-propelled over long distances through optofluidic photonic crystal fibers and their deformation due to shear forces monitored.
 More than 40 years ago, the foundation for optical tweezers was laid when Arthur Ashkin demonstrated that near the focus of a laser beam, momentum transfer between light and dielectric particles creates gradient forces large enough to pull the particle into the center of highest intensity and scattering forces that push it in the propagation direction of the beam. Optical trapping of microparticles and cells can be established either by balancing the axial forces of two weakly-focused counter-propagating beams or by using a single tightly focused laser beam. These optical tweezers have developed into an important tool in cell biological research. Optical tweezers can be used not only to fix cells during manipulation but also to investigate the interconnection of a cell’s elasticity to its physiology: healthy and diseased cells differ notably in their mechanical responses, prominent examples being blood disorders, asthma and cancer.
More than 40 years ago, the foundation for optical tweezers was laid when Arthur Ashkin demonstrated that near the focus of a laser beam, momentum transfer between light and dielectric particles creates gradient forces large enough to pull the particle into the center of highest intensity and scattering forces that push it in the propagation direction of the beam. Optical trapping of microparticles and cells can be established either by balancing the axial forces of two weakly-focused counter-propagating beams or by using a single tightly focused laser beam. These optical tweezers have developed into an important tool in cell biological research. Optical tweezers can be used not only to fix cells during manipulation but also to investigate the interconnection of a cell’s elasticity to its physiology: healthy and diseased cells differ notably in their mechanical responses, prominent examples being blood disorders, asthma and cancer.
Researchers from Max Planck Institute for the Science of Light, Erlangen, Germany now report a new tool for biomechanical studies of individual cells: Single red blood cells were laser-propelled through stationary liquid in a microfluidic channel over distances of up to 24 cm. Shear forces on the cell surface result in its deformation. This causes changes in speed that can conveniently be monitored using a non-imaging laser Doppler-velocimetric technique. Numerical simulations allowed the scientists to derive the optical force acting on different cell shapes.
The unique method is based on a liquid-filled hollow-core photonic crystal fiber which provides low-loss light guidance in a well-defined single mode, resulting in highly uniform optical trapping and propulsive forces in the core which at the same time acts as a microfluidic channel. Cells are trapped laterally at the center of the core, several microns away from the glass interface, which eliminates adherence effects and external perturbations.
Dynamic changes in velocity at constant optical powers up to 350 mW indicated stress-induced changes in the shape of the cells, which was confirmed by bright-field microscopy. The deformations in the moving cells were not only due to heating. Even at moderate temperature, notable deformations could be detected, especially for osmotically swollen red blood cells. Interestingly, the deformations occur over timescales of minutes which is rather slow compared to other cell rheological techniques. Re-arrangements of the cytoskeleton might be involved.
The scientists are currently aiming at studying suspended eukaryotic (cancer) cells. These cells are typically ellipsoidal in shape and more rigid than red blood cells, which prevents them from undergoing peculiar changes in shape. Simulations of the optical forces would be possible, allowing for a complete theoretical analysis of the system. Beyond that, the method may find applications in on-chip cell transport. Cells might be held stationary against a mild counterflow carrying precise amounts of medical drugs. Moreover, cell-cell interactions between suspended cells might be studied.