What happens in the liver during cholestasis? A novel in vivo imaging technique based on fluorescence microscopy delivers insight into the hepatic excretory function in rat.
Bile excretion is a major function of the liver to remove endogenous compounds such as bilirubin, and xenobiotics such as drugs from circulation. An impairment of the involved transport system results in an impairment of bile flow. This phenomenon – known as cholestasis – is associated with an accumulation of potentially toxic substances in the liver and the systemic circulation.
Only few techniques for in vivo imaging of hepatobiliary excretory function have been reported so far. A team led by Michael Bauer from Jena University Hospital (Germany) now reports a new technique based on intravital microscopy, which has a great potential to differentiate between physiological and pathophysiological, i.e. cholestatic, conditions.
The scientists first visualized hepatocellular transport by use of indocyanine green (ICG), an established dye with solely hepatobiliary excretion, which is applied in the clinical setting for assessment of excretory liver function. In order to optimize visualization and to expand the possibilities of analysis, the researchers tested an additional dye: DY635, a benzopyrylium based hemocyanine dye. This dye was chosen due to its binding capacity to plasma proteins and its relatively lipophilic character. In their study, Bauer and his team validated the dye for assessing hepatic excretory function. A primarily hepatic clearance of DY635 was confirmed by fluorophotometric determination of the dye in blood, bile and urine of rats.
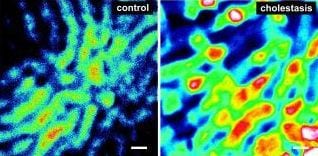
For the study cholestasis was induced in rats by infection. The dyes were administered via the jugular vein catheter. Left liver lobes were examined by intravital microscopy using an inverted epifluorescence microscope. The researchers observed that ICG was delivered from the portal triad and distributed in sinusoids. While ICG was transported through the sinusoids towards the central vein, the dye was taken up by hepatocytes. Thereby, uptake exceeded excretion, resulting in a hepatocellular accumulation of ICG even in healthy animals. Under these conditions, the bile canaliculi could not be clearly visualized. In contrast to ICG, transcellular transport of DY635 was much faster, enabling a clear visualization of the bile canaliculi.
In cholestatic animals, hepatobiliary excretion of DY635 markedly differed from control animals. Intravital fluorescence microscopy of the liver revealed higher dye concentrations in the cholestatic liver. Thereby, DY635 could discriminate hepatic excretory dysfunction in cholestatic as opposed to control animals. Subsequent image analysis revealed that the dye accumulated within distinct regions, presumably single hepatocytes with an average size of 20–30 mm, predominantly around the central veins. These results are consistent with the fact that inflammation-induced down-regulation of canalicular transporters is most pronounced in pericentral hepatocytes.
DY635 even enabled an analysis at high resolution suggesting hepatocyte uncoupling as well as failure of primarily the canalicular pole, allowing in vivo insights into molecular mechanisms of this critical facet of hepatobiliary function.
The new approach may help to understand the effect of different forms of cholestasis. In addition, ICG and DY635 can be used as surrogates for various drugs enabling study of pharmocokinetics and tracking of xenobiotics.