 Production of hydrogen fuel by electrolysis or photolysis of water is a promising and sustainable solution to energy problems and the development of novel electrocatalysts has challenged scientists. Numerous materials have been developed and applied as electrocatalysts and photocatalysts in the hydrogen evolution reaction (HER). Among them, Pt is one of the best electrocatalysts in the HER, but the scarcity and high cost of Pt limit its practical applications. To realize future industrial applications in solar hydrogen production, more efficient and cost effective electrocatalysts are required.
Production of hydrogen fuel by electrolysis or photolysis of water is a promising and sustainable solution to energy problems and the development of novel electrocatalysts has challenged scientists. Numerous materials have been developed and applied as electrocatalysts and photocatalysts in the hydrogen evolution reaction (HER). Among them, Pt is one of the best electrocatalysts in the HER, but the scarcity and high cost of Pt limit its practical applications. To realize future industrial applications in solar hydrogen production, more efficient and cost effective electrocatalysts are required.
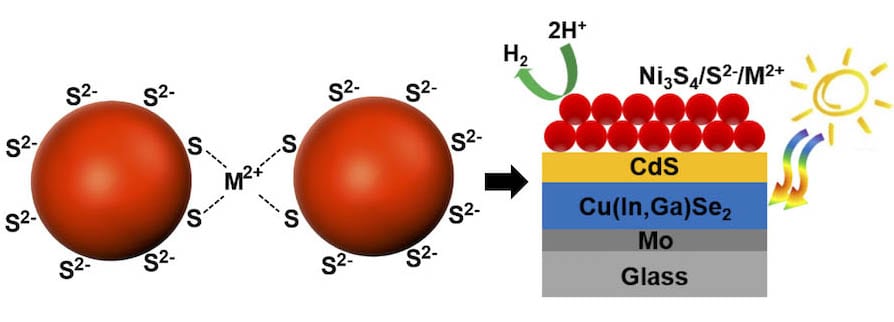
In ChemNanoMat, researchers from Kyoto University, The University of Tokyo, and others, Japan, have reported the formation of layer-by-layer (LbL) assembled cocatalyst films of S2–-stabilized Ni3S4 nanoparticles (NPs) for HER in the Artificial Photosynthesis Project of the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
They have demonstrated that S2–-stabilized Ni3S4 NPs films could be assembled on fluorine-doped tin oxide electrodes and photoelectrodes by a LbL process with metal cation linkers under mild conditions. For both electrodes, the LbL assembled films of the S2–-stabilized Ni3S4 NPs showed higher photocurrents and current densities with increasing light irradiation time. The activation was attributed to a structural transformation of Ni3S4 into Ni(POx)y during the HER, which contributed to the high and stable (photo)current densities of the LbL assembled films. This LbL assembly process could be applicable to forming ligand-free and robust NP films for many types of photoelectrodes without deteriorating their intrinsic optical properties.
Text kindly provided by the authors.