Natural gas is responsible for about 25% of the global energy supply. It has tremendous calorific value and a growing reputation as an alternative fuel with a lower global warming potential than coal and oil. Yet natural gas is not just hydrocarbon in nature, it is mixed with significant quantities of nitrogen (N2) and carbon dioxide (CO2), which degrades its heating value.
If the methane (CH4) in natural gas could be separated at high purity from the N2 and CO2 diluents in an energy- and cost-efficient process, its worth as a fuel would be greatly enhanced.
Although this purification task sounds straightforward, it turns out to be scientifically challenging and technologically demanding to achieve by traditional cryogenic distillation methods. In this technology, gaseous species are liquified at high pressure and low temperature and separated based on the differences in their boiling points. This process is expensive in energy and complex to implement at scale.
To circumvent these complications, the differences in the size and shape of CH4, N2, and CO2 can, in principle, be beneficially exploited by literally sieving them through nanometer-sized pores of a membrane whose dimensions and geometry have been judiciously tailored through aperture engineering chemistry to selectively pass N2 and CO2, but block CH4.
The snag is the chemical and physical similarities of CH4 to N2 and CO2 with respect to its inertness, critical molecular dimensions, polarizability, and boiling point, making the development of an energy- and cost-efficient separation process especially tricky. Knowledge of critical molecular sizes are pivotal for understanding selective molecular exclusion by pores of different size and shape.
While the concepts and principles underpinning selective separation of gaseous mixtures for a wide range of nanoporous materials — carbons, zeolites, and molecular sieves — have been well-documented and industrialized at-scale, none can achieve the energy and economic performance metrics required to separate CH4 from N2 and CO2 mixtures.
MOF to the rescue
This situation experienced a dramatic change for the better very recently, with the announcement of a novel class of metal organic framework (MOF) materials — the size and shape of its nanoscale pores had been creatively tailored to achieve this demanding task with exquisite fidelity.
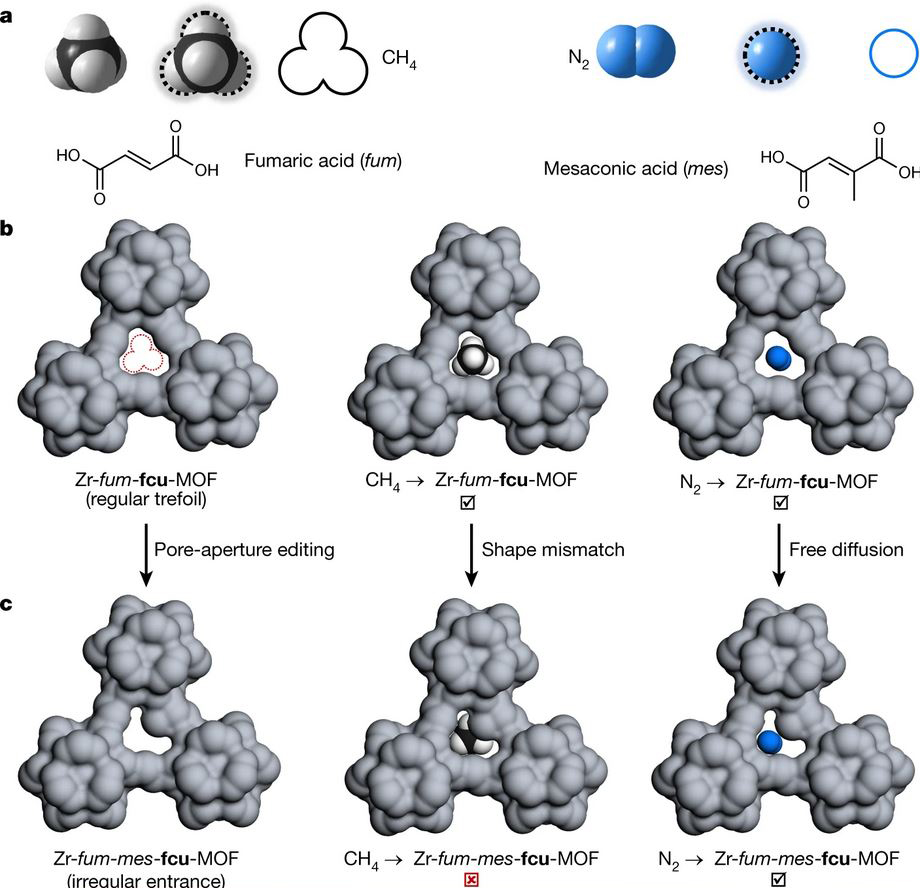
This remarkable accomplishment was achieved in a MOF in which the inorganic building blocks based on a zirconium oxide cluster were connected and assembled with two very slightly geometrically different organic linkers: a fumarate (fum) and a mesaconate (mes) in a 2:1 2:1 to form Zr-fum67-mes33-fcu-MOF.
The entire scheme to achieve this goal is illustrated in the figure below, which shows how shape-mismatch between CH4 and N2, introduced by pore-aperture editing, enables their separation by molecule-scale shape differences. Similar ideas applied to CH4 and CO2.
One can see that the pore aperture of this chemically tailored MOF had the ideal size and shape specifically designed for the efficient removal of the N2 and CO2 from natural gas. To be more precise, molecular asymmetry was intentionally introduced into the pore aperture of the MOF.
This was accomplished in Zr-fum67-mes33-fcu-MOF through creative organic linker design such that it blocked the diffusive transport of the tetrahedral CH4 molecule into the pore mouth, yet permitted linear N2 and CO2 to infuse within.
The separation selectivity achieved for CH4 from N2 and CO2 proved to be a world-record under practical operating pressures, and the system was amenable to scaling to industrial relevant extents.
A techno-economic analysis showed that membranes fabricated from the MOF had the potential to significantly reduce CH4 purification energy intensity and costs in natural gas for both N2 removal, as well as the simultaneous removal of N2 and CO2 compared to cryogenic distillation and a mine-based CO2 capture.
On the economics of the process, the MOF improved the price of purification for the CH4/N2 and CH4/N2/CO2 separations by 66% and 73% respectively relative to cryogenic distillation and amine-based CO2 capture.
What could be more natural than that accomplishment for natural gas! The story does not end with natural gas as this rational approach to pore aperture edited MOF membranes can be applied to other binary gas mixtures, including H2/CH4, CO2/CH4, H2/N2 and CO2/N2, thereby providing a universal paradigm for creating high fidelity molecule selective MOF sieves.
Reference: Mohamed Eddaoudi, et al., Asymmetric pore windows in MOF membranes for natural gas valorization, Nature (2022). DOI: 10.1038/s41586-022-04763-5

















