Around 1 in 250 pregnancies result in identical or “monozygotic” twins, but the mechanism behind monozygotic twinning has remained elusive, partly because the phenomenon is so rare and partly due to ethical concerns surrounding the study of human embryos.
A group of researchers have recently developed a model to study monozygotic twinning outside the womb, avoiding the use of natural human embryos. The model consists of embryo-like structures called blastoids, bundles of cells that resemble blastocysts formed in the early stage of pregnancy. During this stage, the blastocyst implants into the wall of the uterus, where it develops into an embryo.
“Blastoids are stem-cell-derived in vitro models of the blastocyst stage of embryogenesis,” explained Dorian Luijkx, a researcher involved in the study who works at the MERLN Institute of Technology-Inspired Regenerative Medicine at Maastricht University, the Netherlands. “Model” is the operative word here — unlike blastocysts, blastoids can’t develop into humans. At least not yet.
To form blastoids, the researchers grew human stem cells in parallel microwells made of polycarbonate film. These microwell arrays, custom-made using a thermoforming process, had thin, transparent walls, allowing the researchers to continuously monitor and image the blastoids in high resolution as they formed.
“Within each round-bottomed microwell, the cells cluster together and form a blastoid,” Luijkx noted. Using this high-throughput screening platform, Luijkx and his colleagues were able to optimize the conditions for growing twin blastoids on a large scale.
“Only because of the parallel growth and upscaling of the blastoid culture were we able to identify this as a relatively common event,” stated Erik Vrij, principal investigator of the study, who’s based in the Department of Gynecology and Obstetrics at Maastricht University Medical Center.
Leading theory of twinning
The researchers found that twin blastoids were more likely to spontaneously form when they seeded a large number of stem cells — up to 300 — in each microwell, making the phenomenon easier to study. To these wells, they also added a high concentration of a chemical that promotes stem cell differentiation into trophectoderm — the cells make up the blastocyst and later form the placenta.
“In our model, we see that the splitting of the inner cell mass [that forms a twin blastoid] happens concurrently with the expansion of the trophectoderm and that boosting trophectoderm expansion [chemically] is leading to an increase in twin blastoids,” Vrij told us.
“Therefore, we hypothesize that a very rapid expansion of the trophectoderm might be a driving factor for inner cell mass splitting,” he added.
Vrij also stressed that whether the expansion of the trophectoderm actually leads to splitting of the inner cell mass depends on the delicate balance between the cohesion among the cells of the inner cell mass and adhesion of these cells to the trophectoderm.
The leading hypothesis for monozygotic twinning is that the inner cell mass splits into two sometime during the first week of fertilization. Depending on the timing of this splitting, four different subtypes of twins can form, including monochorionic twins, which share a placenta and account for 75% of all identical twins.
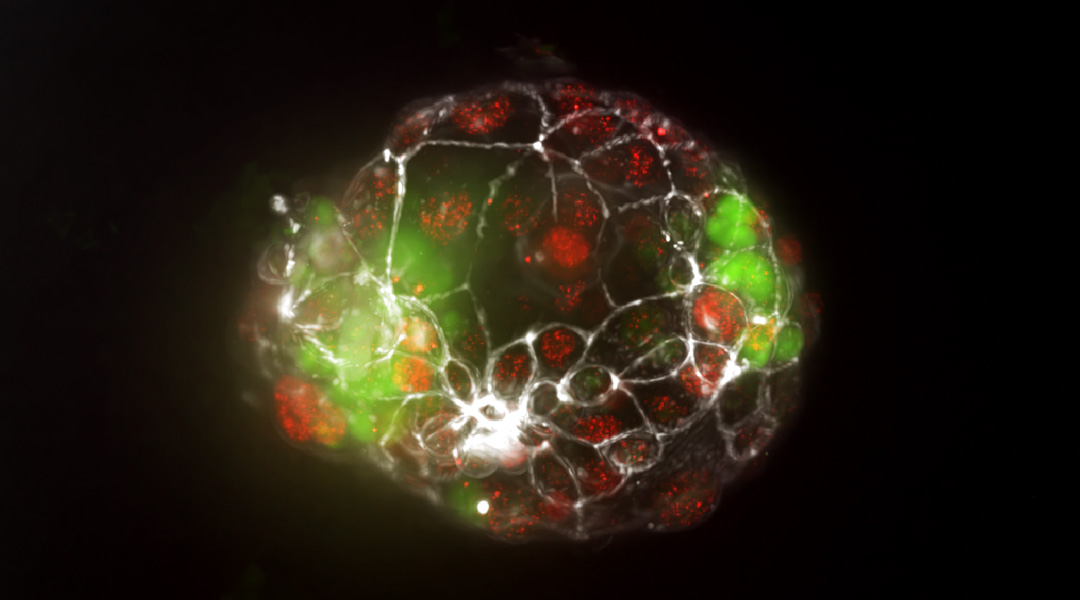
“The exact timing of this splitting — right after fertilization, during the blastocyst stage, or right after implantation — dictates the subtype of the monozygotic twin,” Vrij explained. “Within our twin blastoids, which we would categorize as monochorionic twins, we observe division of the inner cell mass during the formation of blastocyst-like structures. This provides strong support for the leading hypothesis of twinning.”
The researchers also found that the twin blastoids and normal “singleton” blastoids that lead to the birth of one baby had the same cell-number ratios of the three cell lineages typical of the blastocyst stage. According to Vrij, this could mean that the embryo self-regulates the appropriate numbers of cells.
Mimicking implantation
To model the implantation of blastocysts in the uterus during pregnancy — twinning can also occur immediately after blastocyst implantation — the researchers co-cultured singleton and twin blastoids in a microfluidic chip for 48 hours. This endometrium-on-a-chip allowed them to test the adhesion of the blastoids to the uterine lining known as the epithelium.
“Most importantly, we see in our newly developed microfluidic assay that twin blastoids have a higher adherence rate on the bioengineered endometrium monolayers than singleton blastoids,” Stefan Giselbrecht, another of the study’s lead investigators at MERLN Institute, pointed out. “This may suggest an advantage for a twin embryo in the implantation stage of development,” he added.
In the future, the researchers plan to use their model to see if twinning rates differ between male and female embryos and to optimize the twin blastoid formation conditions for another cell line and compare them to those of the stem cells used in this study. They’d also like to better understand the twinning mechanism in order to potentially reduce complications associated with assisted reproductive technologies like in vitro fertilization.
“We aim to delve deeper into the molecular and biophysical mechanisms that underlie the splitting of the inner cell mass, as these may give us clues as to how twinning rates in assisted reproductive technology can be reduced or completely avoided,” Giselbrecht shared. He’s referring to the 2–12 times higher chance of identical twins being born with assisted reproductive technologies, which increase the risk of pregnancy complications.
Vrij and his colleagues are also interested in studying how chemicals that find their way into the environment, like endocrine disruptors, affect embryo implantation.
At this stage, the researchers are also unsure to what extent their experimental setup and steps they took to promote blastoid growth affect the rate of twinning. Some aspects of the study clearly deviate from the conditions of a naturally developing embryo.
“The deviation from [natural embryo] development is that we do not start with a fertilized egg cell that goes through several rounds of cell division first before differentiation to the different cell types of the early embryo,” Vrij commented.
Although these questions need to be addressed, the model is an important starting point for studying the formation and development of twin embryos.
Reference: Dorian G. Luijkx, et al. Monochorionic Twinning in Bioengineered Human Embryo Model, Advanced Materials (2024). DOI: 10.1002/adma.202313306
Feature image: Fluorescent image of a monochorionic twin blastoid